Answered step by step
Verified Expert Solution
Question
1 Approved Answer
b) What was the initialreactant concentration for the reaction described in PartA? c) The reactant concentration in a first-orderreaction was 9.60?10 ?2 M after30.0 ss
 b) What was the initialreactant concentration for the reaction described in PartA?
b) What was the initialreactant concentration for the reaction described in PartA?
c) The reactant concentration in a first-orderreaction was 9.60?10?2 M after30.0 ss and 7.80?10?3 M after90.0 s . What is the rate constant for thisreaction?
d) The reactant concentration in a second-orderreaction was 0.330 M after 265 ss and3.10?10?2 M after 865 s . What isthe rate constant for this reaction?
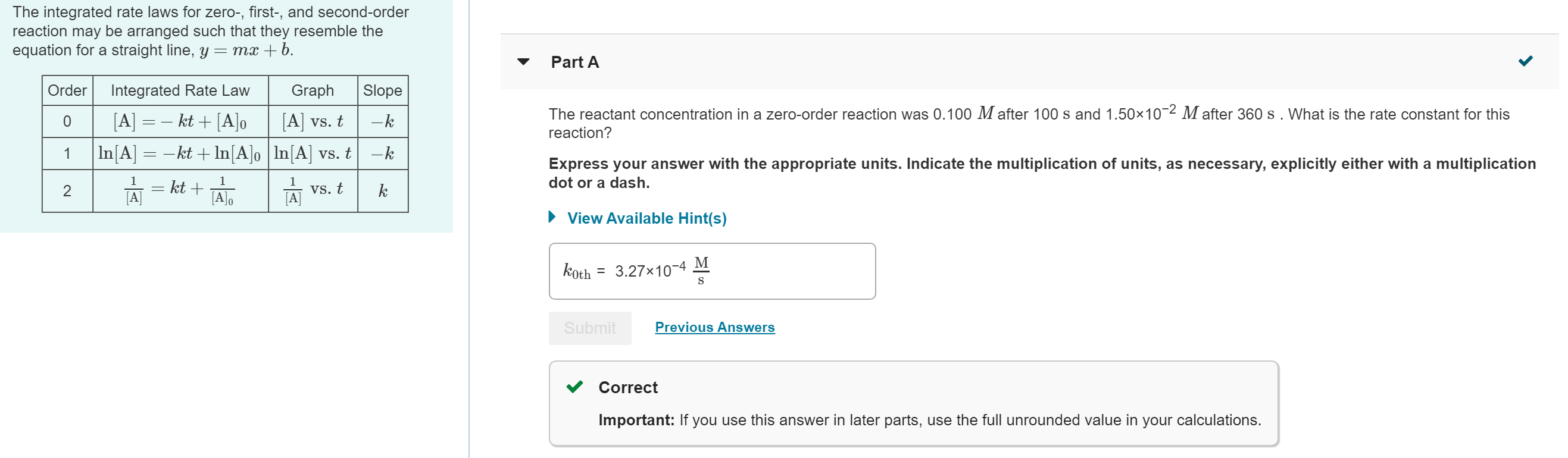
The integrated rate laws for zero-, first-, and second-order reaction may be arranged such that they resemble the equation for a straight line, y = mx +b. Order 0 1 2 Integrated Rate Law Graph [A] vs. t [A] = kt + [A]o |ln[A] = kt + ln [A]o | ln[A] vs. t 1 1 vs. t [A]o [A] 1 = kt + Slope - k -k k Part A The reactant concentration in a zero-order reaction was 0.100 M after 100 s and 1.5010- Mafter 360 s. What is the rate constant for this reaction? Express your answer with the appropriate units. Indicate the multiplication of units, as necessary, explicitly either with a multiplication dot or a dash. View Available Hint(s) koth 3.27x10-4 Submit M S Previous Answers Correct Important: If you use this answer in later parts, use the full unrounded value in your calculations.
Step by Step Solution
★★★★★
3.49 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


