Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat,
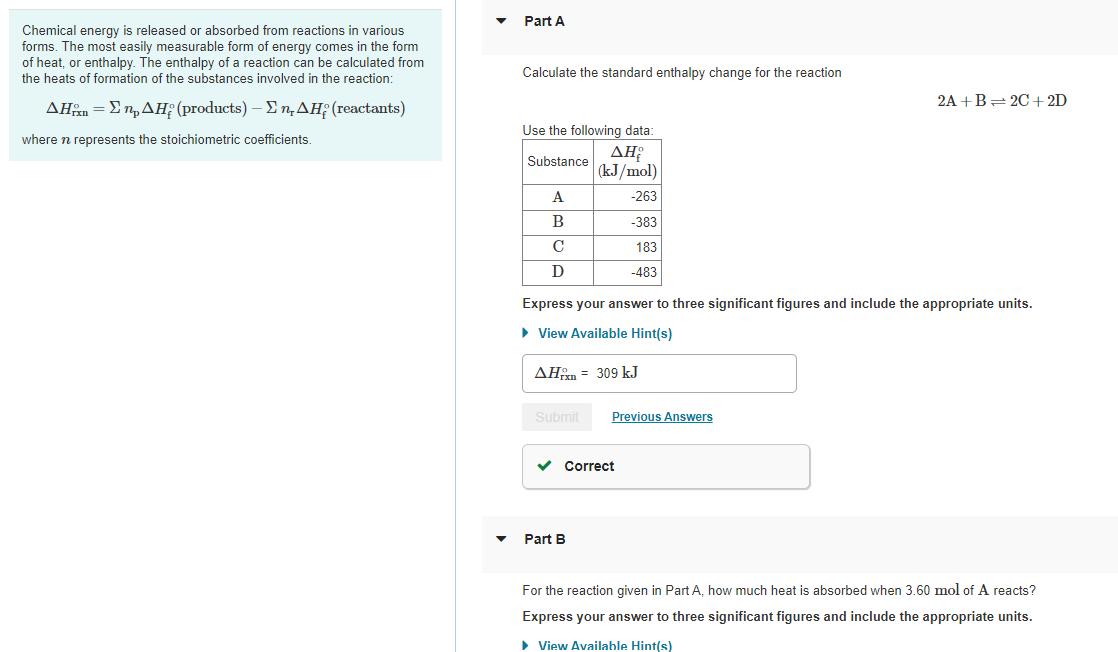
Chemical energy is released or absorbed from reactions in various forms. The most easily measurable form of energy comes in the form of heat, or enthalpy. The enthalpy of a reaction can be calculated from the heats of formation of the substances involved in the reaction: n = (products) - . (reactants) where n represents the stoichiometric coefficients. Part A Calculate the standard enthalpy change for the reaction Use the following data: Substance (kJ/mol) A B C D Express your answer to three significant figures and include the appropriate units. View Available Hint(s) AH 309 kJ Submit -263 -383 183 -483 Correct Part B Previous Answers 2A+B=2C+2D For the reaction given in Part A, how much heat is absorbed when 3.60 mol of A reacts? Express your answer to three significant figures and include the appropriate units. View Available Hint(s)
Step by Step Solution
★★★★★
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
PARTA PARTB As 2 A 2B A H...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


