Question
B6H6 is an octahedral boron cluster. Synthetically it is feasible to replace part of the hydrogen (H) atoms with deuterium (D). The structures of
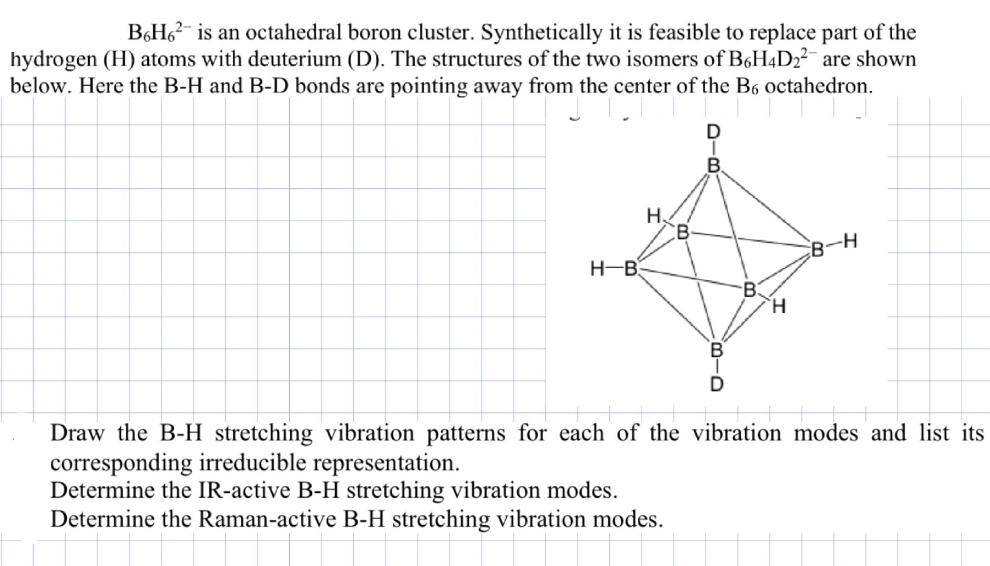
B6H6 is an octahedral boron cluster. Synthetically it is feasible to replace part of the hydrogen (H) atoms with deuterium (D). The structures of the two isomers of B6H4D22 are shown below. Here the B-H and B-D bonds are pointing away from the center of the B6 octahedron. H-B H. B H B-H Draw the B-H stretching vibration patterns for each of the vibration modes and list its corresponding irreducible representation. Determine the IR-active B-H stretching vibration modes. Determine the Raman-active B-H stretching vibration modes.
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
BH Stretching Vibration Patterns and Activities in B6H4D22 Isomers Heres the analysis of BH stretchi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Thomas Engel, Philip Reid
3rd edition
805338423, 080533842X, 978-0321812001
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



