Answered step by step
Verified Expert Solution
Question
1 Approved Answer
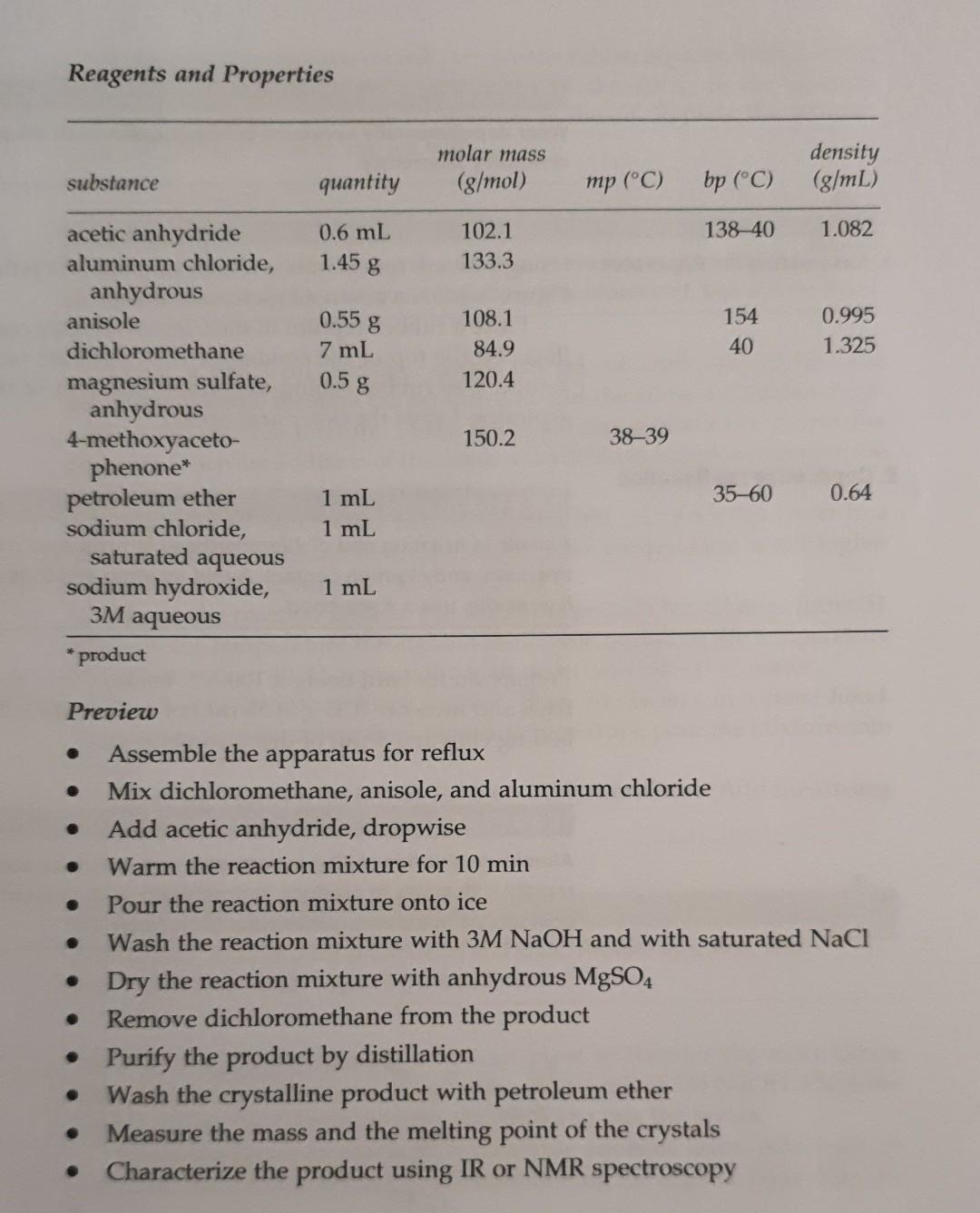
[background] please provide calculations. The topic of this experiment is a Friedel-Crafts actylation. Reagents and Properties molar mass (g/mol) density (g/mL) substance quantity mp (C)

[background]
please provide calculations.
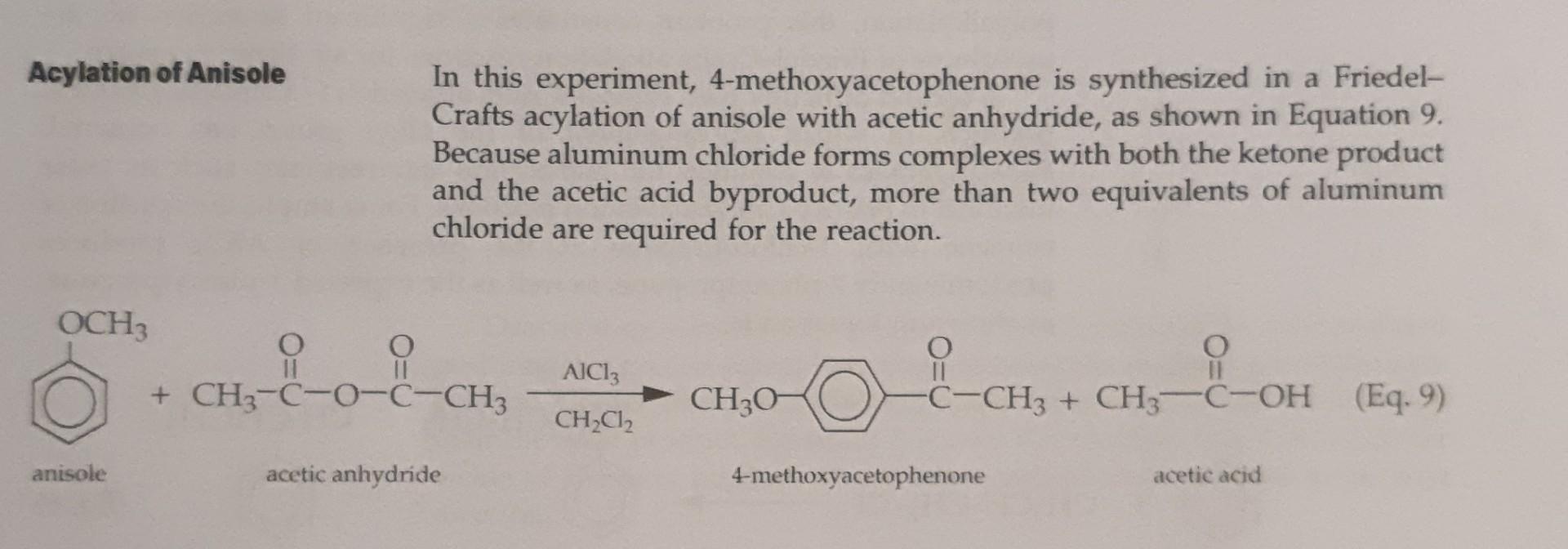
The topic of this experiment is a Friedel-Crafts actylation.
Reagents and Properties molar mass (g/mol) density (g/mL) substance quantity mp (C) bp (C) 0.6 mL 138-40 1.082 102.1 133.3 1.45 g 0.55 g 108.1 84.9 120.4 154 40 0.995 1.325 7 ml 0.5 g acetic anhydride aluminum chloride, anhydrous anisole dichloromethane magnesium sulfate, anhydrous 4-methoxyaceto- phenone* petroleum ether sodium chloride, saturated aqueous sodium hydroxide, 3M aqueous * product 150.2 38-39 35-60 0.64 1 mL 1 mL 1 mL * Preview . . Assemble the apparatus for reflux Mix dichloromethane, anisole, and aluminum chloride Add acetic anhydride, dropwise Warm the reaction mixture for 10 min Pour the reaction mixture onto ice Wash the reaction mixture with 3M NaOH and with saturated NaCl Dry the reaction mixture with anhydrous MgSO4 Remove dichloromethane from the product Purify the product by distillation Wash the crystalline product with petroleum ether Measure the mass and the melting point of the crystals Characterize the product using IR or NMR spectroscopy . 4. Calculate the theoretical yield, in grams, of 4-methoxyacetophenone that can be produced in this experiment. Record your result here and in your laboratory notebook. Acylation of Anisole In this experiment, 4-methoxyacetophenone is synthesized in a Friedel- Crafts acylation of anisole with acetic anhydride, as shown in Equation 9. Because aluminum chloride forms complexes with both the ketone product and the acetic acid byproduct, more than two equivalents of aluminum chloride are required for the reaction. OCH Il AlCl3 + CH3-C-0-C-CH3 CH3O Oc+, I -C-CH3 + CH3-C-OH (Eq. 9 CH2Cl2 anisole acetic anhydride 4-methoxyacetophenone acetic acidStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


