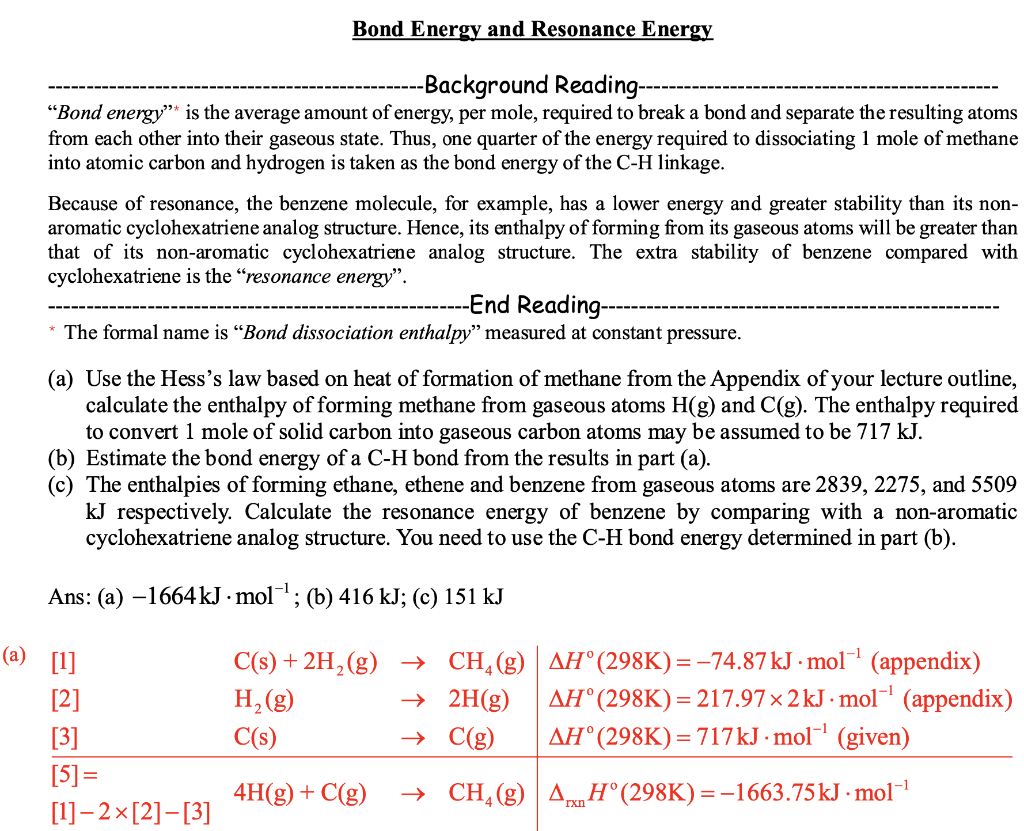
Background Reading- "Bond energy" is the average amount of energy, per mole, required to break a bond and separate the resulting atoms from each other into their gaseous state. Thus, one quarter of the energy required to dissociating 1 mole of methane into atomic carbon and hydrogen is taken as the bond energy of the C - H linkage. Because of resonance, the benzene molecule, for example, has a lower energy and greater stability than its nonaromatic cyclohexatriene analog structure. Hence, its enthalpy of forming from its gaseous atoms will be greater than that of its non-aromatic cyclohexatriene analog structure. The extra stability of benzene compared with cyclohexatriene is the "resonance energy". -----------------------------------------------End Reading * The formal name is "Bond dissociation enthalpy" measured at constant pressure. (a) Use the Hess's law based on heat of formation of methane from the Appendix of your lecture outline, calculate the enthalpy of forming methane from gaseous atoms H(g) and C(g). The enthalpy required to convert 1 mole of solid carbon into gaseous carbon atoms may be assumed to be 717kJ. (b) Estimate the bond energy of a C-H bond from the results in part (a). (c) The enthalpies of forming ethane, ethene and benzene from gaseous atoms are 2839,2275 , and 5509 kJ respectively. Calculate the resonance energy of benzene by comparing with a non-aromatic cyclohexatriene analog structure. You need to use the C-H bond energy determined in part (b). Ans: (a) 1664kJmol1; (b) 416kJ; (c) 151kJ \begin{tabular}{llll|l} {[1]} & C(s)+2H2(g) & & CH4(g) & H(298K)=74.87kJmol1 (appendix) \\ {[2]} & H2(g) & & 2H(g) & Ho(298K)=217.972kJmol1 (appendix) \\ {[3]} & C(s) & & C(g) & Ho(298K)=717kJmol1 (given) \\ \hline[5]= & & & CH4(g) & rxnH(298K)=1663.75kJmol1 \end{tabular} Background Reading- "Bond energy" is the average amount of energy, per mole, required to break a bond and separate the resulting atoms from each other into their gaseous state. Thus, one quarter of the energy required to dissociating 1 mole of methane into atomic carbon and hydrogen is taken as the bond energy of the C - H linkage. Because of resonance, the benzene molecule, for example, has a lower energy and greater stability than its nonaromatic cyclohexatriene analog structure. Hence, its enthalpy of forming from its gaseous atoms will be greater than that of its non-aromatic cyclohexatriene analog structure. The extra stability of benzene compared with cyclohexatriene is the "resonance energy". -----------------------------------------------End Reading * The formal name is "Bond dissociation enthalpy" measured at constant pressure. (a) Use the Hess's law based on heat of formation of methane from the Appendix of your lecture outline, calculate the enthalpy of forming methane from gaseous atoms H(g) and C(g). The enthalpy required to convert 1 mole of solid carbon into gaseous carbon atoms may be assumed to be 717kJ. (b) Estimate the bond energy of a C-H bond from the results in part (a). (c) The enthalpies of forming ethane, ethene and benzene from gaseous atoms are 2839,2275 , and 5509 kJ respectively. Calculate the resonance energy of benzene by comparing with a non-aromatic cyclohexatriene analog structure. You need to use the C-H bond energy determined in part (b). Ans: (a) 1664kJmol1; (b) 416kJ; (c) 151kJ \begin{tabular}{llll|l} {[1]} & C(s)+2H2(g) & & CH4(g) & H(298K)=74.87kJmol1 (appendix) \\ {[2]} & H2(g) & & 2H(g) & Ho(298K)=217.972kJmol1 (appendix) \\ {[3]} & C(s) & & C(g) & Ho(298K)=717kJmol1 (given) \\ \hline[5]= & & & CH4(g) & rxnH(298K)=1663.75kJmol1 \end{tabular}







