Answered step by step
Verified Expert Solution
Question
1 Approved Answer
based from the work given I need a claim and evidence and reasoning argument 3. The Table below presents data relevant to the flasks that

based from the work given I need a claim and evidence and reasoning argument
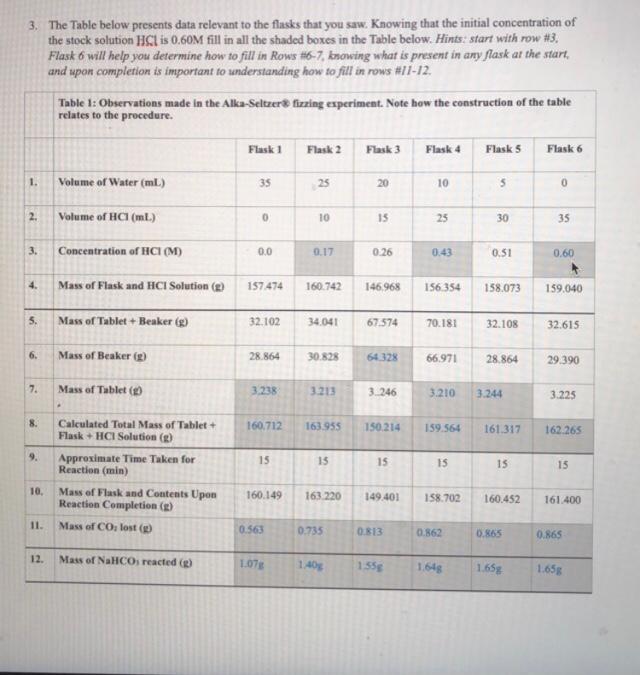
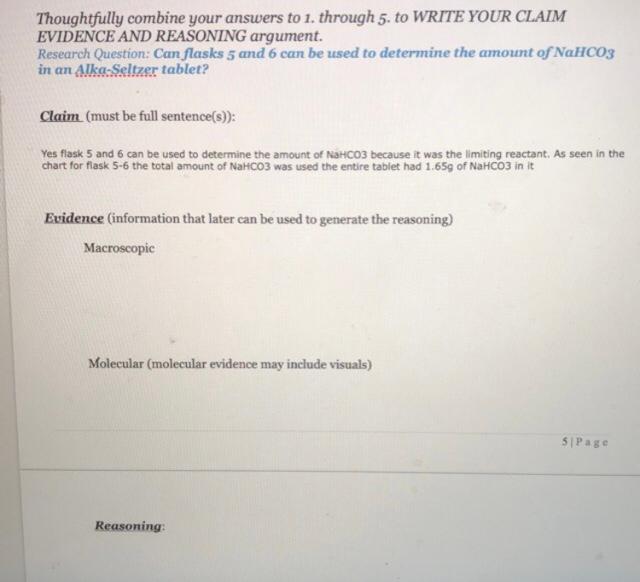
3. The Table below presents data relevant to the flasks that you saw. Knowing that the initial concentration of the stock solution HCl is 0.60M fill in all the shaded boxes in the Table below. Hints: start with row #3. Flask 6 will help you determine how to fill in Rows #6-7, knowing what is present in any flask at the start and upon completion is important to understanding how to fill in rows #11-12. Table 1: Observations made in the Alka-Seltzer fizzing experiment. Note how the construction of the table relates to the procedure Flask 1 Flask 2 Flask 3 Flask 4 Flask 5 Flask 6 1. Volume of Water (ml.) 35 25 20 10 5 0 2. Volume of HC (ml.) 0 10 15 25 30 35 3. Concentration of HCI (M) 0.0 0.17 0.26 0.43 0.51 0.60 4 Mass of Flask and HCI Solution (2) 157474 160.742 146968 156 354 158.073 159.040 5. Mass of Tablet +Beaker (8) 32.102 34.041 67.574 70.181 32.108 32.615 6. Mass of Beaker (2) 28.864 30.828 64328 66.971 28.864 29.390 7. Mass of Tablet 3.238 3.213 3.246 3.210 3.244 3.225 8. Calculated Total Mass of Tablet + Flask + HCI Solution (D) 160,712 163.955 150.214 159.564 161.317 162.265 9. 15 15 15 IS 15 15 Approximate Time Taken for Reaction (min) Mass of Flask and Contents Upon Reaction Completion (g) 10. 160.149 163 220 149.401 158.702 160.452 161.400 11. Mass of CO, lost () 0.563 0.735 0.813 0.862 0.865 0.865 12. Mass of NaHCOs reacted () 1.07 1.405 1558 1648 1.65g 1.658 4. How would you connect the observations you recorded to the entries in Table 1? ie. How do you relate what you "saw" in the video to entries in the table? So based from what we saw the from the volume of water decrease from flask 1 to flask 6 and the volume of HCL increased in the table and as we go up the number of finsk the solution becomes more acidic and from the table we can see flask 6 with the most amount of HCL with no water and the darkest colour which was the one with the lowest ph value compared to flask 1 with thew highest amount of water and lowest amount of HCL making it the lowest acidic solution with a more lighter colour with the highest ph value. 5. How will you determine which flasks have HCl in excess? Hint, will the observations alone tell you or do you need information from the Table given? We cannot determine the excess HCl through observation alone. As from the table we can see that the amount of Na2CO3 that is depleted doesn't increase after flask 5 but the amount of HCl that is added increases in flask 6 which signifies that the solution has excess acid which doesn't react with anything else. Thoughtfully combine your answers to 1. through 5. to WRITE YOUR CLAIM EVIDENCE AND REASONING argument. Research Question: Can flasks 5 and 6 can be used to determine the amount of NaHCO3 in an Alka-Seltzer tablet? Claim (must be full sentence(s)): Yes flask 5 and 6 can be used to determine the amount of NaHCO3 because it was the limiting reactant. As seen in the chart for flask 5-6 the total amount of NaHCO3 was used the entire tablet had 1.659 of NaHCO3 in it Evidence (information that later can be used to generate the reasoning) Macroscopic Molecular (molecular evidence may include visuals) 5 Page ReasoningStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


