Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Based on Lewis structure, what is the structure of ICls indicate if the molecule is polar or not? of Select one: a. trigonal bipyramidal, non-polar
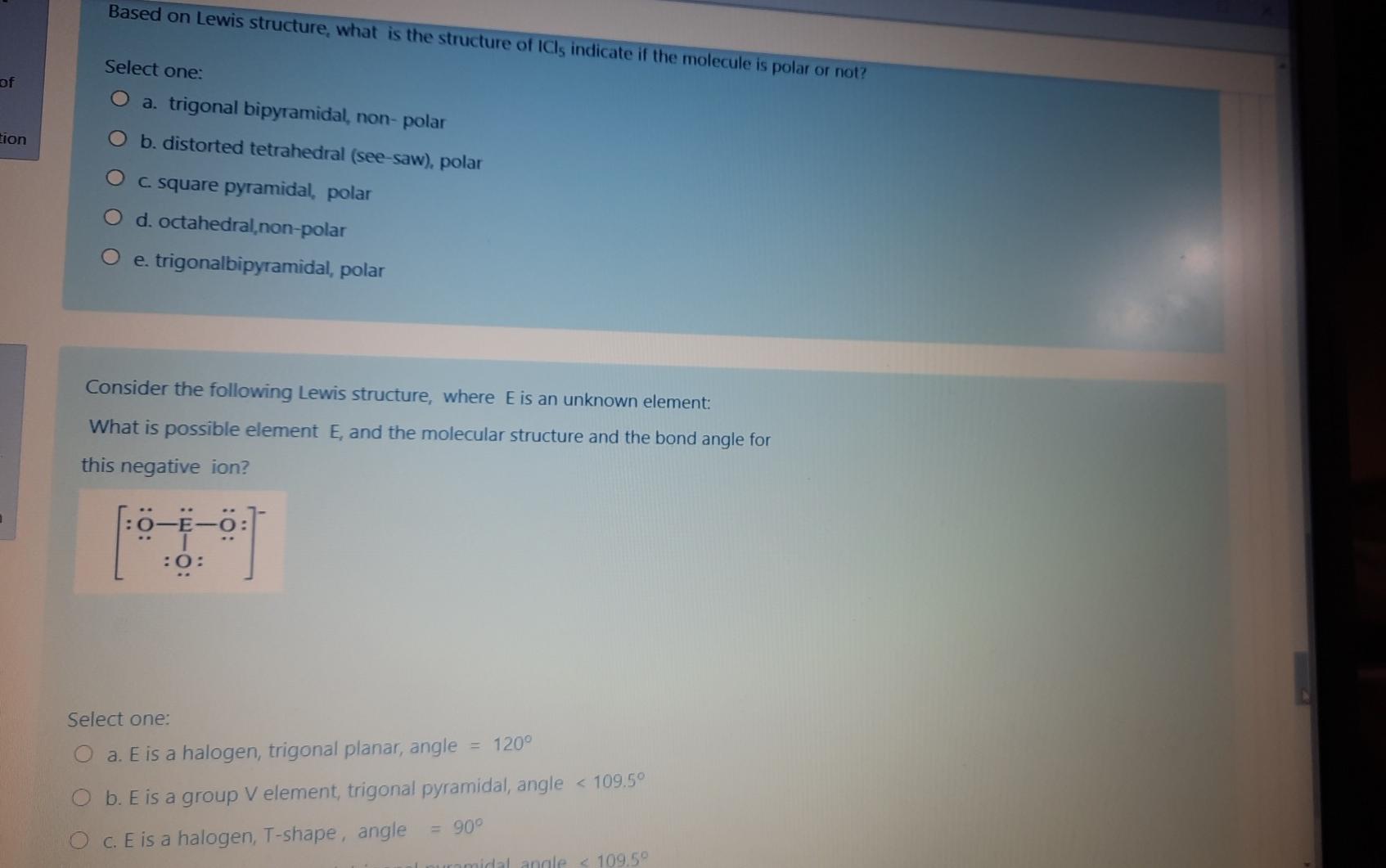
Based on Lewis structure, what is the structure of ICls indicate if the molecule is polar or not? of Select one: a. trigonal bipyramidal, non-polar b. distorted tetrahedral (see-saw), polar tion c. square pyramidal, polar d. octahedral, non-polar O e. trigonalbipyramidal, polar Consider the following Lewis structure, where E is an unknown element: What is possible element E, and the molecular structure and the bond angle for this negative ion? 20: Select one: O a. E is a halogen, trigonal planar, angle 120 O b. E is a group V element, trigonal pyramidal, angle
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


