Question
Based on the following data, what is the Br-Br bond energy? 2 H2(g) + Br2(g) HBr(g); AH=-36.44 kJ/mol-rxn Bond Bond Energy (kJ/mol) - 435
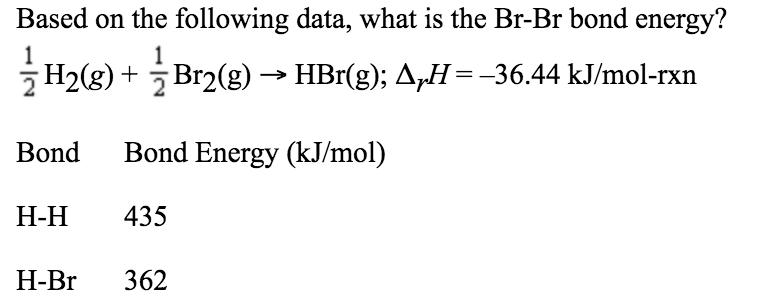
Based on the following data, what is the Br-Br bond energy? 2 H2(g) + Br2(g) HBr(g); AH=-36.44 kJ/mol-rxn Bond Bond Energy (kJ/mol) - 435 -Br 362
Step by Step Solution
There are 3 Steps involved in it
Step: 1
dH rxn BE of reactanats BE of pro...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial Accounting for the Hospitality Industry
Authors: Lea R. Dopson, David K. Hayes
2nd edition
978-1-119-2996, 1119299659, 978-1119386223
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



