Based on the information presented in the attached pictures
Prepare recommendations for a Cleaning Validation study
And
Produce a draft Cleaning Validation protocol to be followed during the validation of the cleaning process for a dual product facility for both LG and DC.
Pay particular attention to the acceptance criteria and rationale for selection.
As part of your study recommendations make comments about any particular elements that might cause you concern and any mitigation measures that could be made.
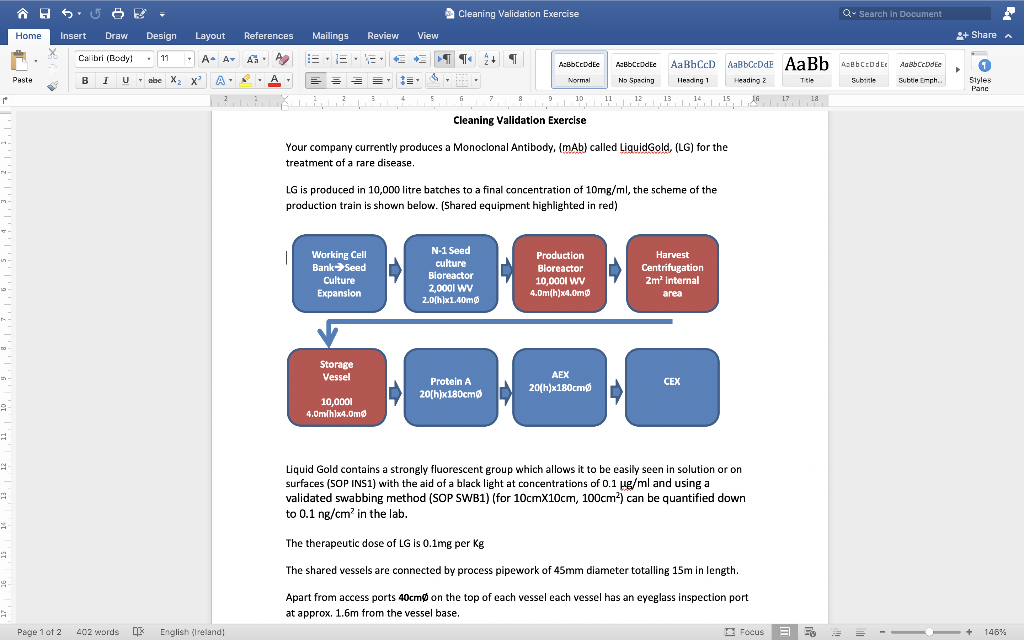
A H 5 US Cleaning Validation Exercise Q- Search in Document Home Insert Draw Design Layout References Mailings Review View 2+ Share A Calibri (Body! . 11 A- AT A 12 I.EE T + 1 Asbbcodec AaBb CODEC No Soscing AaBhCcD ABCDE AaBb4ebtcodec AaBbccdde Paste B 1 U abe X2 X A. * . Normal Heading 2 This Subtitle subtle Emph. Herding 1 14 Styles Panc r 10 11 12 13 15 16 17 18 Cleaning Validation Exercise Your company currently produces a Monoclonal Antibody, (mAb) called LiquidGold, (LG) for the treatment of a rare disease. LG is produced in 10,000 litre batches to a final concentration of 10mg/ml, the scheme of the production train is shown below. (Shared equipment highlighted in red) Working Cell Bank Seed Culture Expansion N-1 Seed culture Bioreactor 2,0001 WV 2.0(h)x1.40mg Production Bioreactor 10,0001 WV 4.Dm(h)x4.0m Harvest Centrifugation 2m internal area AEX Protein A Storage Vessel 10,0001 4.Omlhlx4.Omd 20(h)x180cm 20(h)x180cm 9 Liquid Gold contains a strongly fluorescent group which allows it to be easily seen in solution or on surfaces (SOP INS1) with the aid of a black light at concentrations of 0.1 vg/ml and using a validated swabbing method (SOP SWB1) (for 10cmx10cm, 100cm) can be quantified down to 0.1 ng/cm in the lab. The therapeutic dose of LG is 0.1mg per kg The shared vessels are connected by process pipework of 45mm diameter totalling 15m in length. Apart from access ports 40cm on the top of each vessel each vessel has an eyeglass inspection port at approx. 1.6m from the vessel base. Page 1 of 2 402 words LE English (Ireland! Focus + 148% A5 Cleaning Validation Exercise Q- Search in Document Home Insert Draw Design Layout References Mailings Review View 2+ Share A Calibri (Body! 11 A- AT A 12 3 + 1 Acbbcodec AaBb CODEC No Soscing AaBhCcD ABCDE AaBb4ebtcodec AaBbccdde Paste B 1 U abe X X A. , Normal Hending 1 Heading 2 Title Subtitle subtle Emph. Styles Panc 5 & 9 10 11 14 15 16 121,1 17 18 The therapeutic dose of LG is 0.1mg per kg - The shared vessels are connected by process pipework of 45mm diameter totalling 15m in length. Apart from access ports 40cm on the top of each vessel each vessel has an eyeglass inspection port at approx. 1.6m from the vessel base. The fixed process pipework, joining the vessels each contain a single welded sampling port reducing to 25mm with a pre-valve length of 75mm. The routine cleaning recipe for shared, Stirred tank bioreactors, process pipework, centrifuges and storage vessels is 30mins circulation of 2% NaOH in Wil at 80C (at a volume amounting to 20% of the System Volume), followed by three successive rinses with WFI at 80C prior to SIP . 22 The company has a product in development (also a mAb) called Dark Cyto, DC which is a high potency cytokine blocker with an expected therapeutic dose of 1 ug/Kg. DC is known to contain a lot of hydrophobic residues and is expected to be quite "sticky" to surfaces and so might present challenges for cleaning. DC is also expected to be cytotoxic to the cells used to culture LG with an LDsa of 5ng/ml. N N 19 Based on the information presented: Prepare recommendations for a Cleaning Validation study Page 1 of 2 402 words LE English (Ireland! Focus + 148% A5 SU Cleaning Validation Exercise Q- Search in Document Home Insert Draw Design Layout References Mailings Review View 2+ Share A Calibri (Body! 11 - A. A A 12 I.EE T + 1 Abbcodec Normal AaBb CODEC No Soscing AaBhCcD ABCDE AaBb4ebtcodec AaBbccdde Paste B 1 U abe X X A. A Hesding 1 Heading 2 Title Subine subtle Emph. Styles Panc 4 5 6 7 & 9 10 11 12 13 14 15 16 17 18 Based on the information presented: Prepare recommendations for a Cleaning Validation study And Produce a draft Cleaning Validation protocol to be followed during the validation of the cleaning process for a dual product facility for both LG and DC. Pay particular attention to the acceptance criteria and rationale for selection. As part of your study recommendations make comments about any particular elements that might cause you concern and any mitigation measures that could be made. Page 2 of 2 402 words CX English (Ireland) Focus 3 + 146% A H 5 US Cleaning Validation Exercise Q- Search in Document Home Insert Draw Design Layout References Mailings Review View 2+ Share A Calibri (Body! . 11 A- AT A 12 I.EE T + 1 Asbbcodec AaBb CODEC No Soscing AaBhCcD ABCDE AaBb4ebtcodec AaBbccdde Paste B 1 U abe X2 X A. * . Normal Heading 2 This Subtitle subtle Emph. Herding 1 14 Styles Panc r 10 11 12 13 15 16 17 18 Cleaning Validation Exercise Your company currently produces a Monoclonal Antibody, (mAb) called LiquidGold, (LG) for the treatment of a rare disease. LG is produced in 10,000 litre batches to a final concentration of 10mg/ml, the scheme of the production train is shown below. (Shared equipment highlighted in red) Working Cell Bank Seed Culture Expansion N-1 Seed culture Bioreactor 2,0001 WV 2.0(h)x1.40mg Production Bioreactor 10,0001 WV 4.Dm(h)x4.0m Harvest Centrifugation 2m internal area AEX Protein A Storage Vessel 10,0001 4.Omlhlx4.Omd 20(h)x180cm 20(h)x180cm 9 Liquid Gold contains a strongly fluorescent group which allows it to be easily seen in solution or on surfaces (SOP INS1) with the aid of a black light at concentrations of 0.1 vg/ml and using a validated swabbing method (SOP SWB1) (for 10cmx10cm, 100cm) can be quantified down to 0.1 ng/cm in the lab. The therapeutic dose of LG is 0.1mg per kg The shared vessels are connected by process pipework of 45mm diameter totalling 15m in length. Apart from access ports 40cm on the top of each vessel each vessel has an eyeglass inspection port at approx. 1.6m from the vessel base. Page 1 of 2 402 words LE English (Ireland! Focus + 148% A5 Cleaning Validation Exercise Q- Search in Document Home Insert Draw Design Layout References Mailings Review View 2+ Share A Calibri (Body! 11 A- AT A 12 3 + 1 Acbbcodec AaBb CODEC No Soscing AaBhCcD ABCDE AaBb4ebtcodec AaBbccdde Paste B 1 U abe X X A. , Normal Hending 1 Heading 2 Title Subtitle subtle Emph. Styles Panc 5 & 9 10 11 14 15 16 121,1 17 18 The therapeutic dose of LG is 0.1mg per kg - The shared vessels are connected by process pipework of 45mm diameter totalling 15m in length. Apart from access ports 40cm on the top of each vessel each vessel has an eyeglass inspection port at approx. 1.6m from the vessel base. The fixed process pipework, joining the vessels each contain a single welded sampling port reducing to 25mm with a pre-valve length of 75mm. The routine cleaning recipe for shared, Stirred tank bioreactors, process pipework, centrifuges and storage vessels is 30mins circulation of 2% NaOH in Wil at 80C (at a volume amounting to 20% of the System Volume), followed by three successive rinses with WFI at 80C prior to SIP . 22 The company has a product in development (also a mAb) called Dark Cyto, DC which is a high potency cytokine blocker with an expected therapeutic dose of 1 ug/Kg. DC is known to contain a lot of hydrophobic residues and is expected to be quite "sticky" to surfaces and so might present challenges for cleaning. DC is also expected to be cytotoxic to the cells used to culture LG with an LDsa of 5ng/ml. N N 19 Based on the information presented: Prepare recommendations for a Cleaning Validation study Page 1 of 2 402 words LE English (Ireland! Focus + 148% A5 SU Cleaning Validation Exercise Q- Search in Document Home Insert Draw Design Layout References Mailings Review View 2+ Share A Calibri (Body! 11 - A. A A 12 I.EE T + 1 Abbcodec Normal AaBb CODEC No Soscing AaBhCcD ABCDE AaBb4ebtcodec AaBbccdde Paste B 1 U abe X X A. A Hesding 1 Heading 2 Title Subine subtle Emph. Styles Panc 4 5 6 7 & 9 10 11 12 13 14 15 16 17 18 Based on the information presented: Prepare recommendations for a Cleaning Validation study And Produce a draft Cleaning Validation protocol to be followed during the validation of the cleaning process for a dual product facility for both LG and DC. Pay particular attention to the acceptance criteria and rationale for selection. As part of your study recommendations make comments about any particular elements that might cause you concern and any mitigation measures that could be made. Page 2 of 2 402 words CX English (Ireland) Focus 3 + 146%









