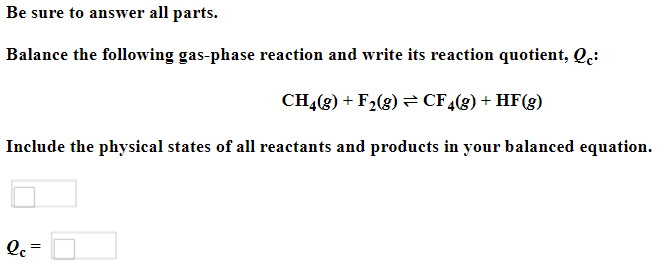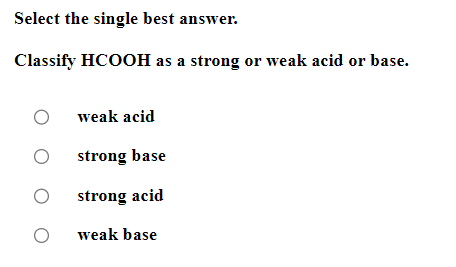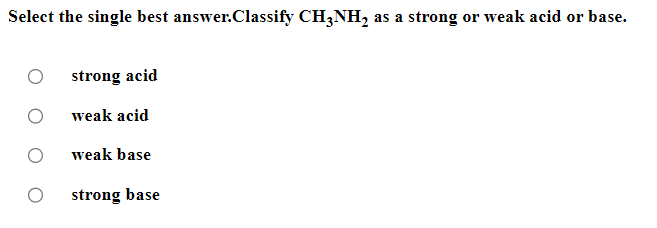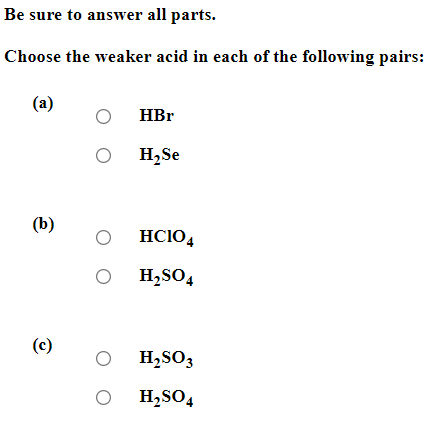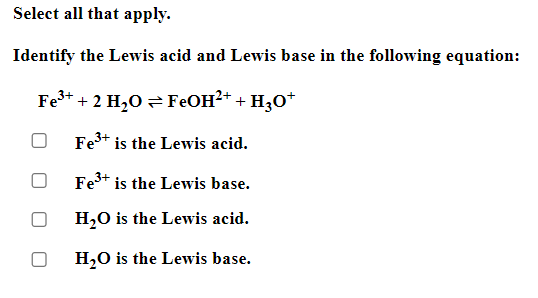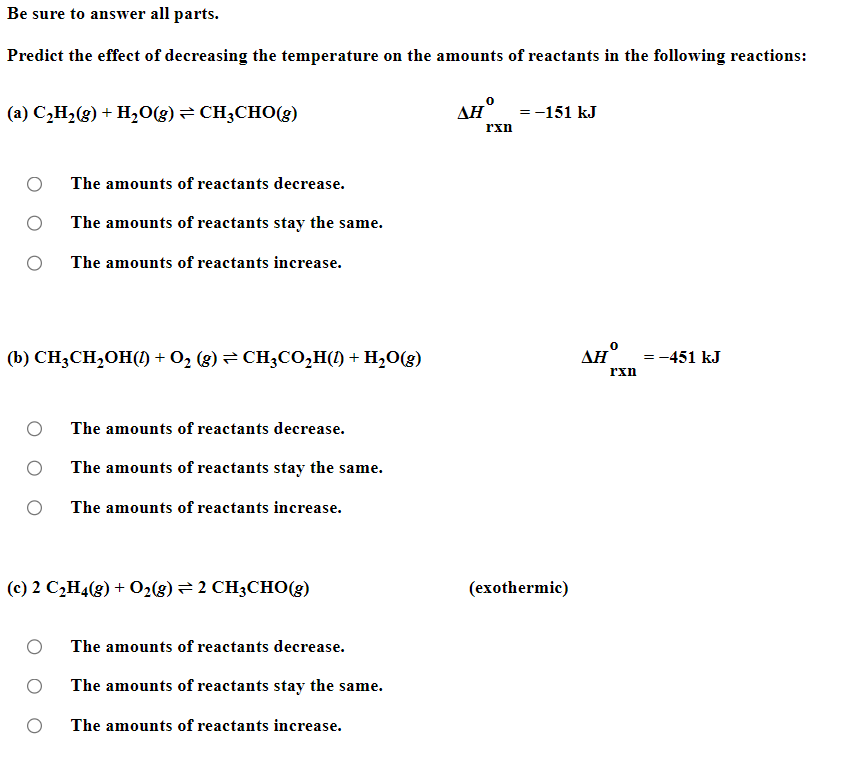


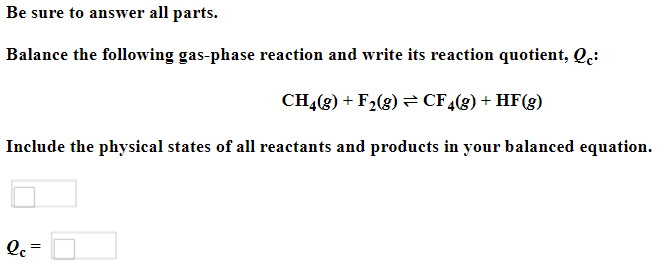








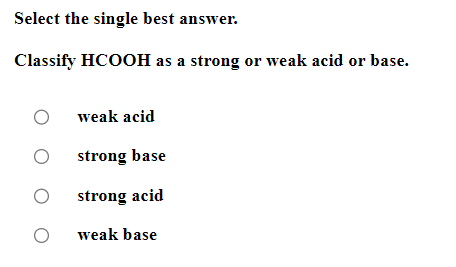
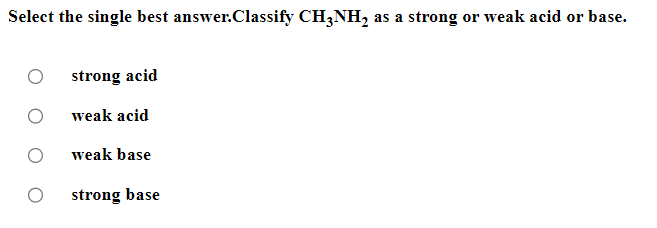
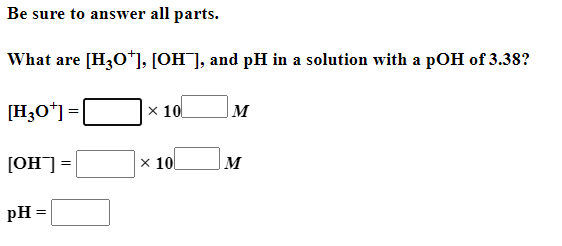



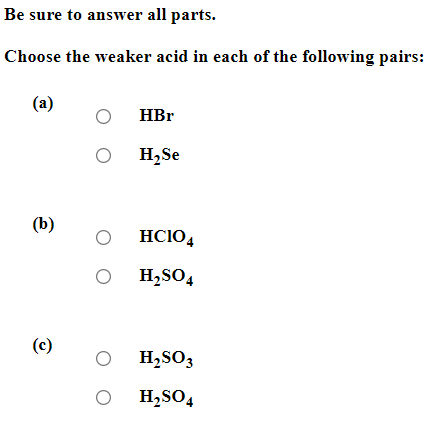
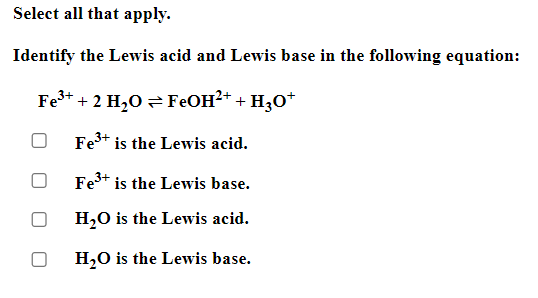

Be sure to answer all parts. Balance the following example of a heterogeneous equilibrium and write its reaction quotient, Qc : S8(s)+F2(g)SF6(g) Include the physical states of all reactants and products in your balanced equation. Qc= Be sure to answer all parts. Predict the effect of decreasing the temperature on the amounts of reactants in the following reactions: (a) C2H2(g)+H2O(g)CH3CHO(g)Hrxn0=151kJ The amounts of reactants decrease. The amounts of reactants stay the same. The amounts of reactants increase. (b) CH3CH2OH(l)+O2(g)CH3CO2H(l)+H2O(g) Hrxn0=451kJ The amounts of reactants decrease. The amounts of reactants stay the same. The amounts of reactants increase. (c) 2C2H4(g)+O2(g)2CH3CHO(g) (exothermic) The amounts of reactants decrease. The amounts of reactants stay the same. The amounts of reactants increase. Be sure to answer all parts. Balance the following reaction and write its reaction quotient, Qc O2(g)O3(g) Include the physical states of all reactants and products in your balanced equation. Qc= Select the single best answer. Sodium bicarbonate undergoes thermal decomposition according to the reaction 2NaHCO3(s)Na2CO3(s)+CO2(g)+H2O(g) How does the equilibrium position shift if 0.20 atm of argon gas is added (assume no change in pressure)? shifts right no change shifts left Be sure to answer all parts. Balance the following gas-phase reaction and write its reaction quotient, Qc : CH4(g)+F2(g)CF4(g)+HF(g) Include the physical states of all reactants and products in your balanced equation Click in the answer box to activate the palette. Write the ion-product expression for silver carbonate. Ksp= Click in the answer box to activate the palette. Write the ion-product expression for barium phosphate. Kcn= Be sure to answer all parts. Calculate the molar solubility of Ca(IO3)2 in each solution below. The Ksp of calcium iodate is 7.1107 (a) 0.060MCa(NO3)2 (b) 0.060MNaIO Click in the answer box to activate the palette. Write the ion-product expression for iron(III) hydroxide. Click in the answer box to activate the palette. Write the ion-product expression for chromium(III) hydroxide. Click in the answer box to activate the palette. Write the ion-product expression for magnesium fluoride. Be sure to answer all parts. What are [H3O+],[OH], and pH in a solution with a pOH of 9.58 ? Make sure your answers are given to the correct number of significant figures. [H3O+]=[OH]=pH=1010MM Be sure to answer all parts. What is the pOH of 2.70MBa(OH)2 solution? Is the solution neutral, acidic, or basic? The pOH of the solution is: The solution is: neutral acidic basic Select the single best answer. Classify HCOOH as a strong or weak acid or base. weak acid strong base strong acid weak base Select the single best answer.Classify CH3NH2 as a strong or weak acid or base. strong acid weak acid weak base strong base Be sure to answer all parts. What are [H3O+],[OH], and pH in a solution with a pOH of 3.38? [H3O+]=[OH]=pH=1010MM Enter your answer in the provided box. Find the pH of a buffer that consists of 0.21M boric acid (H3BO3) and 0.97M sodium borate (NaH2BO3)(pKa of boric acid =9.24) pH= Be sure to answer all parts. Use the dissociation (ionization) constants (Ka) from your textbook to choose the solution with the higher pH in each of the following pairs: (a) 0.1MNiCl2 0.1MNaCl (b) 0.1MSn(NO3)2 0.1MCo(NO3)2 Be sure to answer all parts. Find the pH during the titration of 20.00mL of 0.1000M butanoic acid, CH3CH2CH2COOH (Ka=1.54105), with 0.1000MNaOH solution after the following additions of titrant. (a) 15.00mL : pH= (b) 20.90mL : pH= (c) 26.00mL : pH= Be sure to answer all parts. Choose the weaker acid in each of the following pairs: (a) HBr H2Se (b) HClO4H2SO4 (c) H2SO3H2SO4 Select all that apply. Identify the Lewis acid and Lewis base in the following equation: Fe3++2H2OFeOH2++H3O+ Fe3+ is the Lewis acid. Fe3+ is the Lewis base. H2O is the Lewis acid. H2O is the Lewis base. Select the best single answer. Which, if any, of the following salts will be neutral in an aqueous solution? KBr NH4I KCN