Answered step by step
Verified Expert Solution
Question
1 Approved Answer
begin{tabular}{|c|c|c|c|l|} hline Specimen & TemperatureofPackCarburizing & SoakTimeofPackCarburizing & Quenching Media & HardnessafterPackCarburizing hline 1018Steel & 880920C & 10 min. & oil & 64
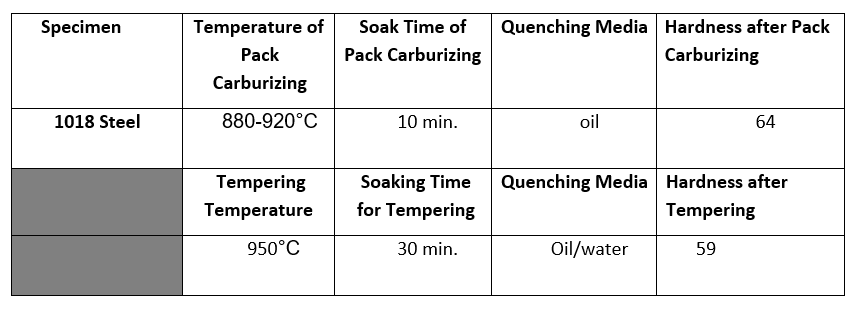

 \begin{tabular}{|c|c|c|c|l|} \hline Specimen & TemperatureofPackCarburizing & SoakTimeofPackCarburizing & Quenching Media & HardnessafterPackCarburizing \\ \hline 1018Steel & 880920C & 10 min. & oil & 64 \\ \hline & TemperingTemperature & SoakingTimeforTempering & Quenching Media & HardnessafterTempering \\ \hline & 950C & 30min. & Oil/water & 59 \\ \hline \end{tabular} 1) Why is this part quenched with water or brine instead of oil or air? 2) Do you think case hardening parts are more ductile than full hardened parts? 3) From the information above, describe how the case hardening process takes place: 4) Does the steel have to be at a temperature above the critical temperature for the case hardening process to commence? 5) From this lab, what deduction can you make about the hardness/ductility relationship? 6) Can this piece be annealed? 7) What element diffuses into the steel? 8) How long does the tempering process take? 9) True or false: I can raise the critical temperature (high heat) to increase the rate of diffusion but take a chance on developing a coarse grain structure. 10) How does pack carburizing differ from induction hardening
\begin{tabular}{|c|c|c|c|l|} \hline Specimen & TemperatureofPackCarburizing & SoakTimeofPackCarburizing & Quenching Media & HardnessafterPackCarburizing \\ \hline 1018Steel & 880920C & 10 min. & oil & 64 \\ \hline & TemperingTemperature & SoakingTimeforTempering & Quenching Media & HardnessafterTempering \\ \hline & 950C & 30min. & Oil/water & 59 \\ \hline \end{tabular} 1) Why is this part quenched with water or brine instead of oil or air? 2) Do you think case hardening parts are more ductile than full hardened parts? 3) From the information above, describe how the case hardening process takes place: 4) Does the steel have to be at a temperature above the critical temperature for the case hardening process to commence? 5) From this lab, what deduction can you make about the hardness/ductility relationship? 6) Can this piece be annealed? 7) What element diffuses into the steel? 8) How long does the tempering process take? 9) True or false: I can raise the critical temperature (high heat) to increase the rate of diffusion but take a chance on developing a coarse grain structure. 10) How does pack carburizing differ from induction hardening Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


