Answered step by step
Verified Expert Solution
Question
1 Approved Answer
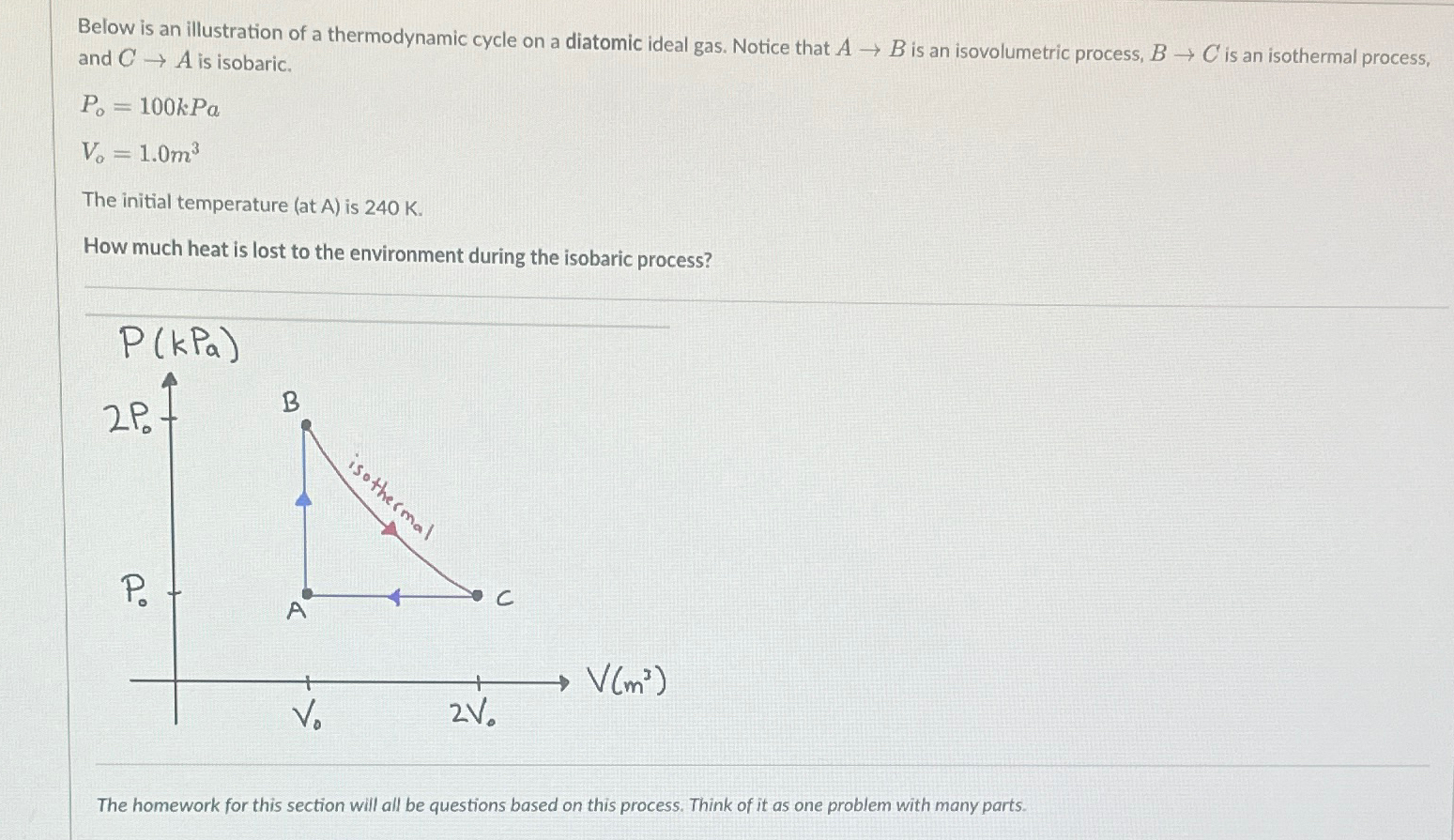
Below is an illustration of a thermodynamic cycle on a diatomic ideal gas. Notice that A B is an isovolumetric process, B C is an
Below is an illustration of a thermodynamic cycle on a diatomic ideal gas. Notice that is an isovolumetric process, is an isothermal process, and is isobaric.
kPa
The initial temperature at A is
How many moles of gas is there?
How much work is done in step
How much heat is transferred in process What is :
How much heat is lost to the environment during the isobaric process?
The homework for this section will all be questions based on this process. Think of it as one problem with many parts.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


