Answered step by step
Verified Expert Solution
Question
1 Approved Answer
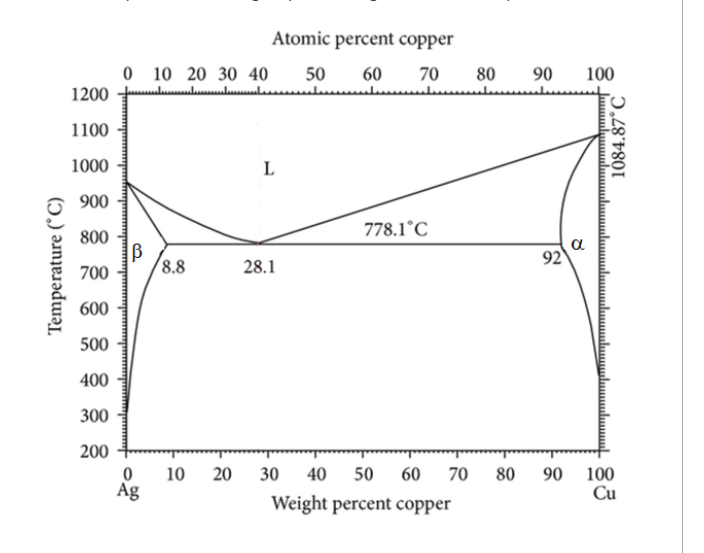
Below is the Binary Phase Diagram for Silver and Copper ( Ag - Cu ) . An alloy is made by mixing 5 grams of
Below is the Binary Phase Diagram for Silver and Copper AgCu An alloy is made by mixing grams of Ag with grams of Cu melted together at oC and then worked at oC After the shape is set, the alloy is cooled to room temperature, which brings out some beta phase from the alloy and strengthens it Atomic percent copper
What is the predicted weight percentage of this beta phase?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


