Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Beta-Carotene shown below is a linear polyene in which 10 single and 11 double bonds alternate along a chain of 22 carbon atoms. Each CC
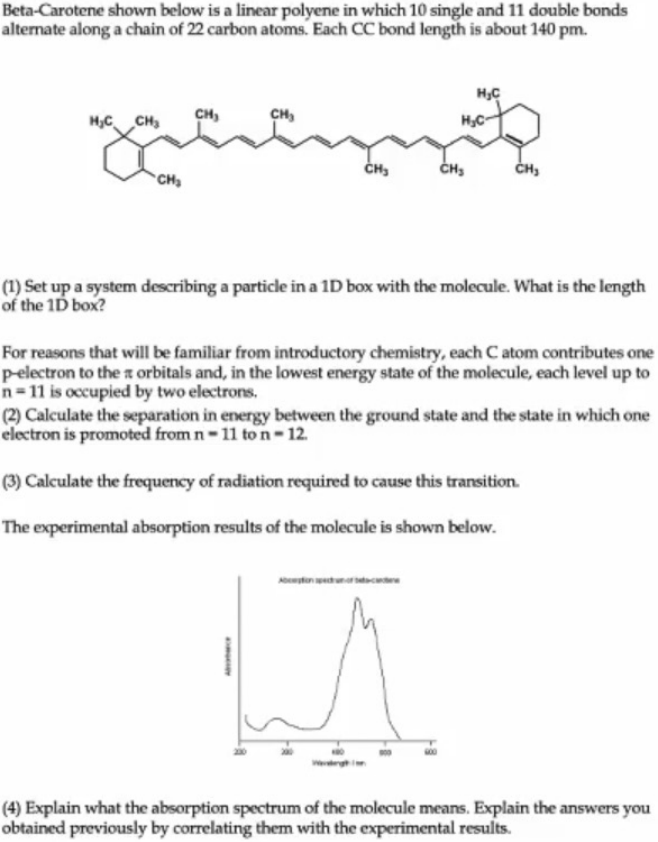 Beta-Carotene shown below is a linear polyene in which 10 single and 11 double bonds alternate along a chain of 22 carbon atoms. Each CC bond length is about 140pm. (1) Set up a system describing a particle in a 1D box with the molecule. What is the length of the 1D box? For reasons that will be familiar from introductory chemistry, each C atom contributes one p-electron to the orbitals and, in the lowest energy state of the molecule, each level up to n=11 is occupied by two electrons. (2) Calculate the separation in energy between the ground state and the state in which one electron is promoted from n=11 to n=12. (3) Calculate the frequency of radiation required to cause this transition. The experimental absorption results of the molecule is shown below. (4) Explain what the absorption spectrum of the molecule means. Explain the answers you obtained previously by correlating them with the experimental results
Beta-Carotene shown below is a linear polyene in which 10 single and 11 double bonds alternate along a chain of 22 carbon atoms. Each CC bond length is about 140pm. (1) Set up a system describing a particle in a 1D box with the molecule. What is the length of the 1D box? For reasons that will be familiar from introductory chemistry, each C atom contributes one p-electron to the orbitals and, in the lowest energy state of the molecule, each level up to n=11 is occupied by two electrons. (2) Calculate the separation in energy between the ground state and the state in which one electron is promoted from n=11 to n=12. (3) Calculate the frequency of radiation required to cause this transition. The experimental absorption results of the molecule is shown below. (4) Explain what the absorption spectrum of the molecule means. Explain the answers you obtained previously by correlating them with the experimental results Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


