Answered step by step
Verified Expert Solution
Question
1 Approved Answer
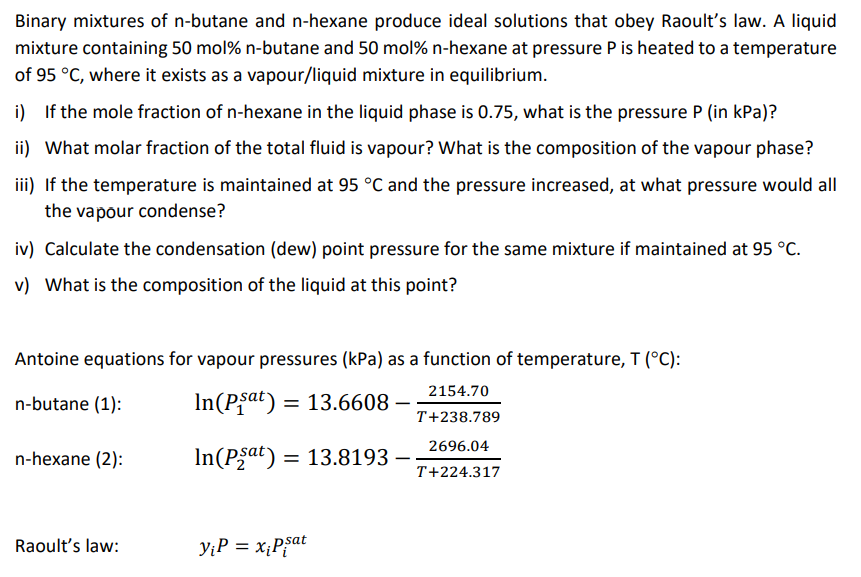
Binary mixtures of n - butane and n - hexane produce ideal solutions that obey Raoult's law. A liquid mixture containing 5 0 mol %
Binary mixtures of butane and hexane produce ideal solutions that obey Raoult's law. A liquid
mixture containing molbutane and molhexane at pressure is heated to a temperature
of where it exists as a vapourliquid mixture in equilibrium.
i If the mole fraction of hexane in the liquid phase is what is the pressure in kPa
ii What molar fraction of the total fluid is vapour? What is the composition of the vapour phase?
iii If the temperature is maintained at and the pressure increased, at what pressure would all
the vapour condense?
iv Calculate the condensation dew point pressure for the same mixture if maintained at
v What is the composition of the liquid at this point?
Antoine equations for vapour pressures as a function of temperature, :
nbutane :
nhexane :
Raoult's law:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


