Answered step by step
Verified Expert Solution
Question
1 Approved Answer
biodiesel and determination of glycerol concentration of sodium thiosulfate is 0.1025M plz answer the question below. 1. Add 50 g of vegetable oil to a
biodiesel and determination of glycerol

concentration of sodium thiosulfate is 0.1025M plz answer the question below.

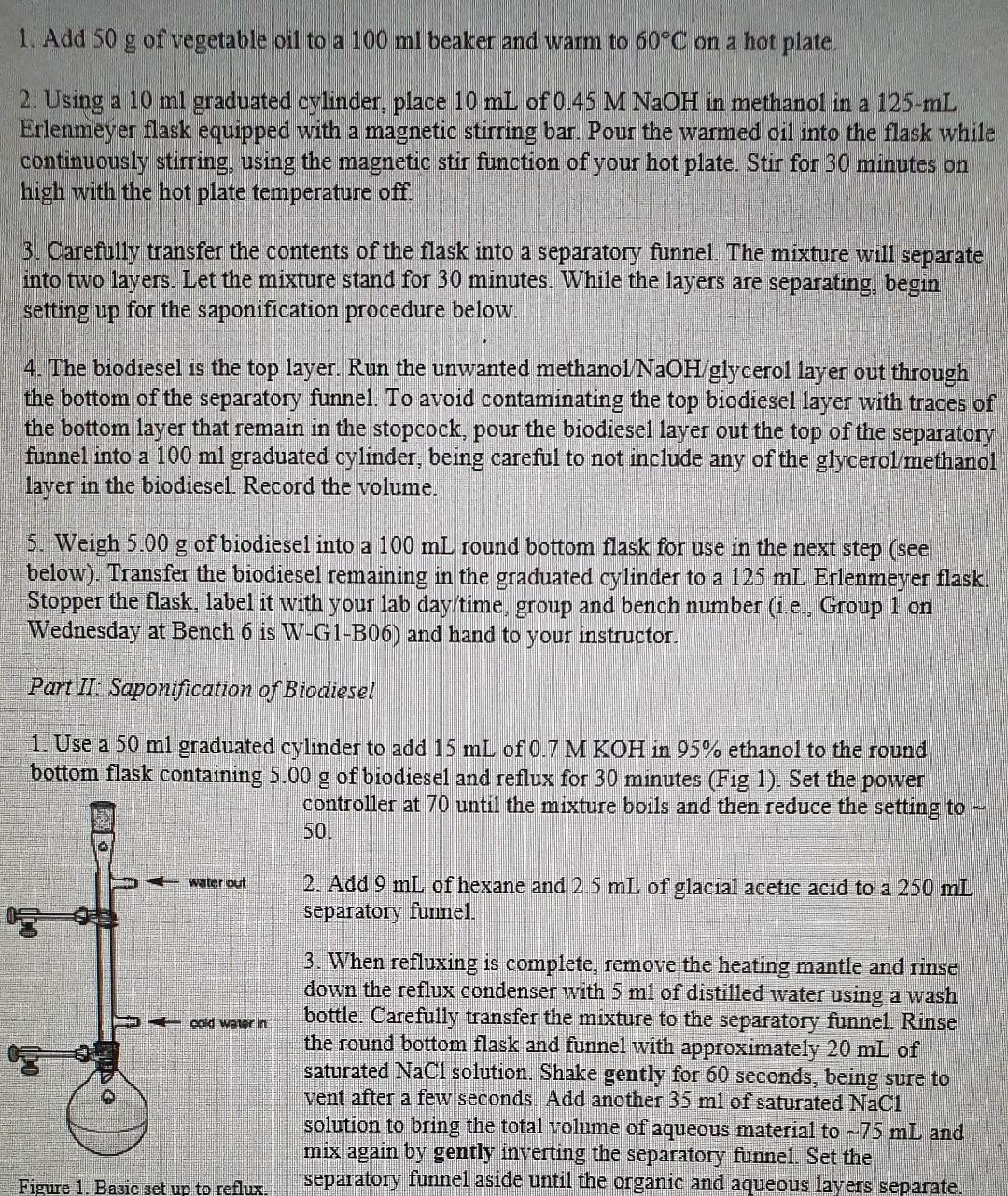
1. Add 50 g of vegetable oil to a 100 ml beaker and warm to 60C on a hot plate. 2. Using a 10 mi graduated cylinder, place 10 ml of 0.45 M NaOH in methanol in a 125-ml Erlenmeyer flask equipped with a magnetic stirring bar. Pour the warmed oil into the flask while continuously stirring, using the magnetic stir function of your hot plate. Stir for 30 minutes on high with the hot plate temperature off. 3. Carefully transfer the contents of the flask into a separatory funnel. The mixture will separate into two layers. Let the mixture stand for 30 minutes. While the layers are separating, begin setting up for the saponification procedure below. 4. The biodiesel is the top layer. Run the unwanted methanol NaOH/glycerol layer out through the bottom of the separatory funnel. To avoid contaminating the top biodiesel layer with traces of the bottom layer that remain in the stopcock, pour the biodiesel yer out the top of the separatory funnel into a 100 ml graduated cylinder, being careful to not include any of the glycerol/methanol layer in the biodiesel. Record the volume. a 5. Weigh 5.00 g of biodiesel into a 100 ml round bottom flask for use in the next step (see below). Transfer the biodiesel remaining in the graduated cylinder to a 125 ml Erlenmeyer flask. Stopper the flask, label it with your lab day/time, group and bench number (i.e., Group 1 on Wednesday at Bench 6 is W-G1-B06) and hand to your instructor. Part II: Saponification of Biodiesel 1. Use a 50 ml graduated cylinder to add 15 ml of 0.7 M KOH in 95% ethanol to the round bottom flask containing 5.00 g of biodiesel and reflux for 30 minutes (Fig 1). Set the power controller at 70 until the mixture boils and then reduce the setting to ~ 50. water out 2. Add 9 mL of hexane and 2.5 mL of glacial acetic acid to a 250 mL separatory funnel. cold water in 3. When refluxing is complete, remove the heating mantle and rinse down the reflux condenser with 5 ml of distilled water using a wash bottle. Carefully transfer the mixture to the separatory funnel. Rinse the round bottom flask and funnel with approximately 20 mL of saturated NaCl solution. Shake gently for 60 seconds, being sure to vent after a few seconds. Add another 35 ml of saturated Naci solution to bring the total volume of aqueous material to ~75 mL and mix again by gently inverting the separatory funnel. Set the separatory funnel aside until the organic and aqueous layers separate. Figure 1. Basic set up to reflux. 4. Recover the aqueous layer in a 125 mL Erlenmeyer flask. Stopper the flask, label it with your lab day/time, group and bench number, using the same code as above, and give it to the instructor Day 2 Part II: Extraction of glycerol from biodiesel 1. Weigh by difference 5.00 g of biodiesel and place in a 250 mL separatory funnel. Using a 50 ml graduate cylinder, add 10 mL of hexane and 20 mL of saturated NaCl solution Stopper and shake gently for 60 seconds (be sure to vent). 2. Add-55 ml of saturated NaCl solution to bring the total volume of aqueous material to 75 ml and mix again by gently inverting the funnel. Set the flask aside until the organic and aqueous layers separate 3. Recover the aqueous layer in a 100 mL volumetric flask. Part IV: Determination of glycerol 1. Transfer the aqueous material from the saponification process (Part II on Day 1) to a 100 mL volumetric flask. To ensure a complete transfer, rinse the Erlenmyer flask that contained the saponified material with ~10 mL of water, add it to the volumetric flask and make up to the mark with water. Shake well and use a volumetric pipet to deliver exactly 20.00 mL into 2 x 250 ml Erlenmeyer flasks. Label the flasks. 2. Add water to the volumetric flask containing the aqueous layer from Part III to make it up to the mark. Shake well and pipet exactly 20.00 mL into 2 X 250 mL Erlenmeyer flasks. Label the flasks 3. Using a graduated cylinder, add -20 mL of distilled water to 2 X 250 Erlenmeyer flasks to serve as blanks 4. Pipet exactly 20.00 mL of periodic acid into each of the 6 X 250 mL Erlenmeyer flasks containing samples and blanks. Mix each container thoroughly and let stand for 10 minutes. These should be colourless. 5. Fill a buret with standardized sodium thiosulfate solution. 6. Using a graduated cylinder, add 10 mL of potassium iodide solution to the first blank solution and let stand for 60 seconds. 7. Add 100 mL of distilled water to the flask, record the volume of sodium thiosulfate solution in the buret to two decimal places and titrate with the standardized sodium thiosulfate solution When the orange color has faded to yellow-orange, use a graduated cylinder to add 2 mL of freshly made starch indicator solution and continue the titration until the blue iodine starch color disappears. Record the final volume of sodium thiosulfate solution in the buret. 8. Repeat steps 6-7 with each of the other 5 flasks. a) Assume that your oil consists entirely of triolein. Determine the limiting reagent in your synthesis of biodiesel. b) If your oil is triolein, draw and name the structure of the only ester that can be produced as the biodiesel product. c) What is the theoretical yield for this reaction? h d) Calculate your actual yield based on the amount of biodiesel you isolated. e) What measures were incorporated into the procedure to attempt to increase yield
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


