Answered step by step
Verified Expert Solution
Question
1 Approved Answer
biodiesel and determination of glycerol: plz answer the question below. Transesterification and Saponification: The reaction to form fatty acid methyl esters uses sodium hydroxide in
biodiesel and determination of glycerol:
plz answer the question below.

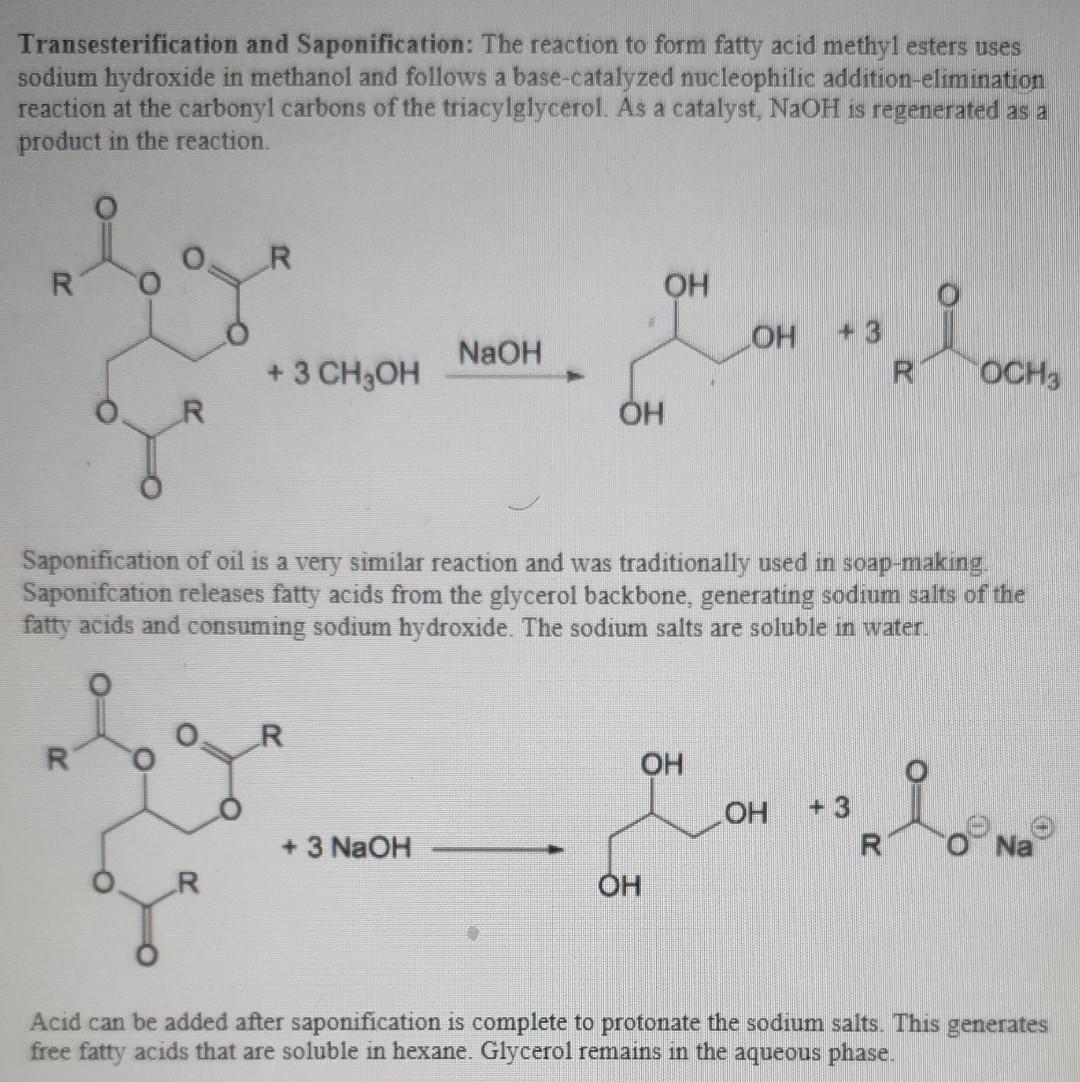
Transesterification and Saponification: The reaction to form fatty acid methyl esters uses sodium hydroxide in methanol and follows a base-catalyzed nucleophilic addition-elimination reaction at the carbonyl carbons of the triacylglycerol. As a catalyst, NaOH is regenerated as a product in the reaction. R OH + 3 NaOH + 3 CH3OH R OCH3 OH Saponification of oil is a very similar reaction and was traditionally used in soap-making. Saponifcation releases fatty acids from the glycerol backbone, generating sodium salts of the fatty acids and consuming sodium hydroxide. The sodium salts are soluble in water. R R OH color OH + 3 R + 3 NaOH Na OH Acid can be added after saponification is complete to protonate the sodium salts. This generates free fatty acids that are soluble in hexane. Glycerol remains in the aqueous phase. Base-catalyzed transesterification follows a nucleophillic substitution mechanism. Write the mechanism for that reaction, showing generation of the nucleophile as the first step. Does it follow an Spl or Sx2 mechanism? Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


