Answered step by step
Verified Expert Solution
Question
1 Approved Answer
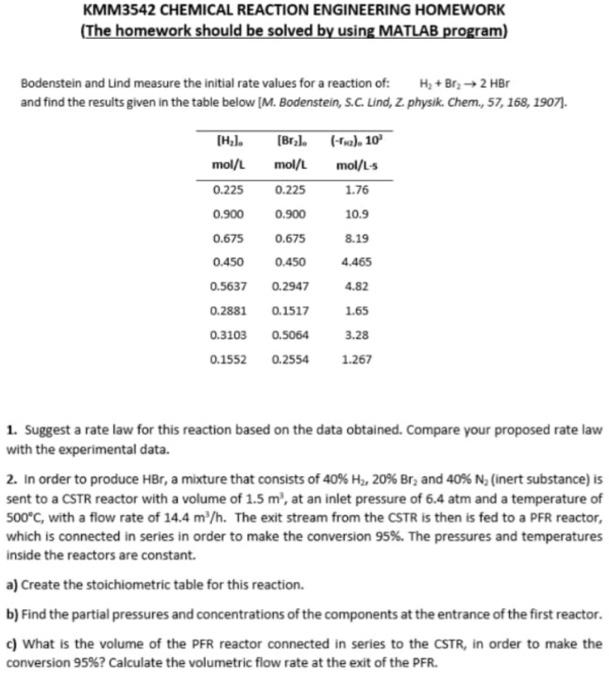
Bodenstein and Lind measure the initial rate values for a reaction of: H, + Br, -> 2 HBr and find the results given in the
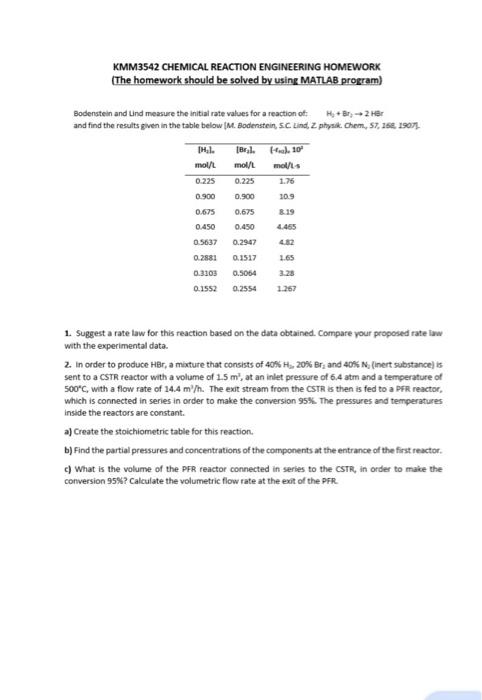
Bodenstein and Lind measure the initial rate values for a reaction of:
H, + Br, -> 2 HBr
and find the results given in the table below [M. Bodenstein, S.C. Lind, 2. physik. Chem., 57, 168, 1907).
TH.1.
mol/L
0.225
0.900
0.675
0.450
0.5637
0.2881
0.3103
0.1552
[Bryl.
(-ric). 10
mol/L mol/L-s
0.225
1.76
0.900
10.9
0.675
8.19
0.450
4.465
0.2947
4.82
0.1517
1.65
0.5064
3.28
0.2554
1.267
- Suggest a rate law for this reaction based on the data obtained. Compare your proposed rate law with the experimental data.
- In order to produce HBr, a mixture that consists of 40% H2, 20% Br, and 40% N, (inert substance) is sent to a CSTR reactor with a volume of 1.5 m', at an inlet pressure of 6.4 atm and a temperature of 500C, with a flow rate of 14.4 m"h. The exit stream from the CSTR is then is fed to a PFR reactor, which is connected in series in order to make the conversion 95%. The pressures and temperatures inside the reactors are constant.
- Create the stoichiometric table for this reaction.
- Find the partial pressures and concentrations of the components at the entrance of the first reactor.
- What is the volume of the PFR reactor connected in series to the CSTR, in order to make the conversion 95%? Calculate the volumetric flow rate at the exit of the PFR.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


