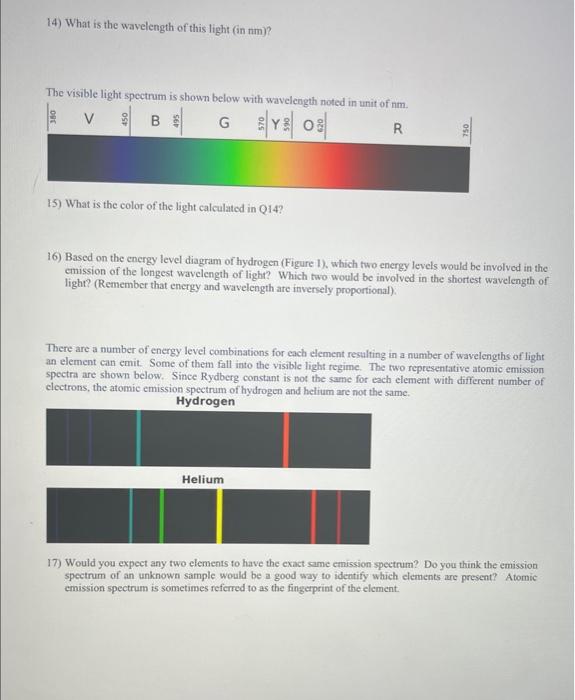
Bohr model as E=RH(31n21), in which RM=2.1791011J is the Rydberg constant for hydrogen and n and n are the final and initial energy levels, respectively 8) What units are used to indicate the energy of each level (n=1,2,3,4,5,6) ? 9) In the diagram, which level is lowest in energy and which is highest in energy? 10) Is the spacing between successive energy levels the same? If not, explain how it changes. 11) In order for an atom to emit (release) light energy, would an clectron need to go from a lower to is higher energy level, or from a higher to a lower energy level? 12) If electrons start in n=6 and jump to n=2, what is the energy of the light (in J) that will be emitted? Please use the Bohr model as described above. The color of the light is dependent on the energy (E) of the light and therefore the wavelength ( , frequency (v). and energy (E) of the light. The relations between these three properties of the light ean be described as: 13) What is the frequency of the light emitted when electrons start in n6 and jump to n=2? Please use the energy difference obtained in Q12. 14) What is the wavelength of this light (in nm)? The visible light spectrum is shown below with waveleneth noted in unit of nm. 15) What is the color of the light calculated in Q14? 16) Based on the energy level diagram of hydrogen (Figure 1), which two energy levels would be involved in the emission of the longest wavelength of light? Which two would be involved in the shortest wavelength of light? (Remember that energy and wavelength are inversely proportional). There are a number of energy level combinations for each element resulting in a number of wavelengths of light an element can emit. Some of them fall into the visible light regime. The two representative atomic emission spectra are shown below. Since Rydberg constant is not the same for each element with different number of electrons, the atomic emission spectrum of hydrogen and helium are not the same. Hydrogen 17) Would you expect any two elements to have the exact same emission spectrum? Do you think the emission spectrum of an unknown sample would be a good way to identify which elements are present? Atomic emission spectrum is sometimes referred to as the fingerprint of the element