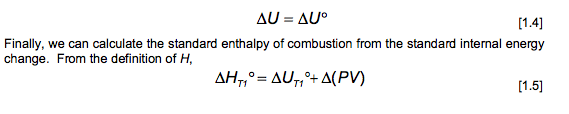Bomb Calorimeter Lab, for Thermodynamics. Please answer all the discussion questions and all calculations!
The theory is:
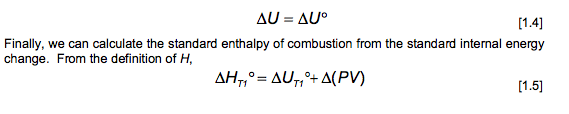
The calculations are:
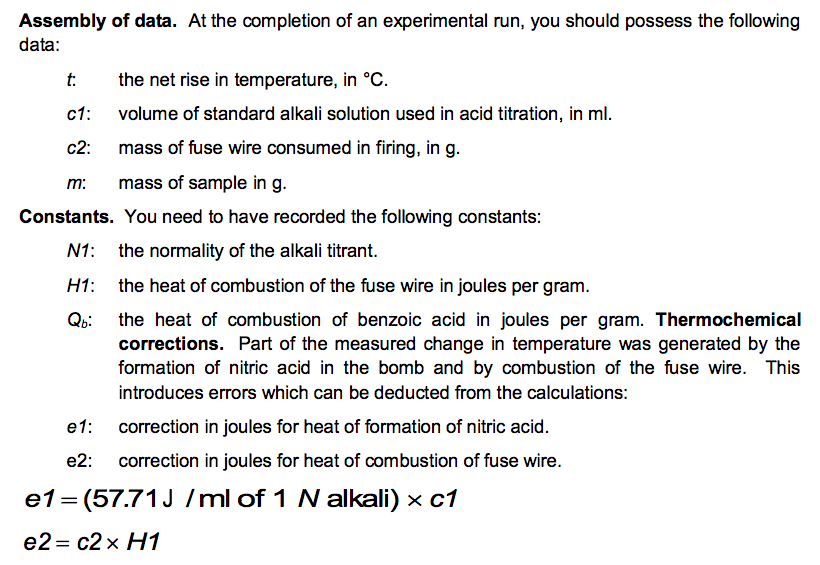
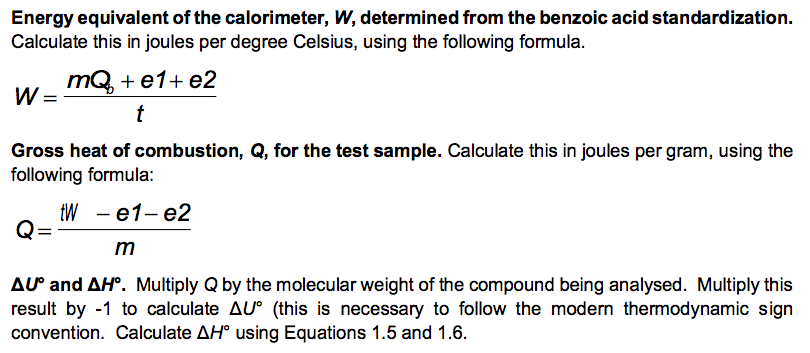

The results are:
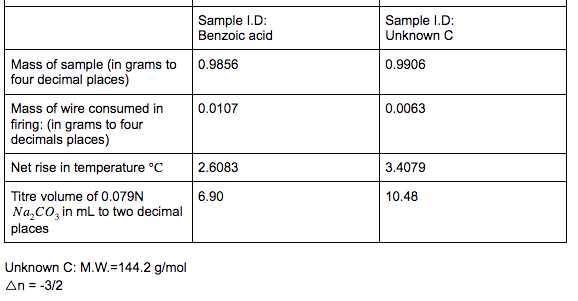
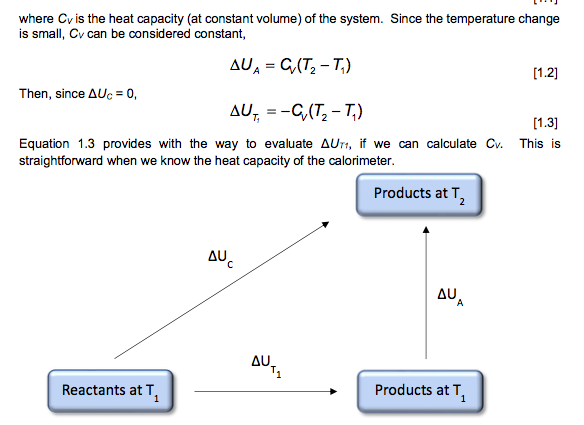
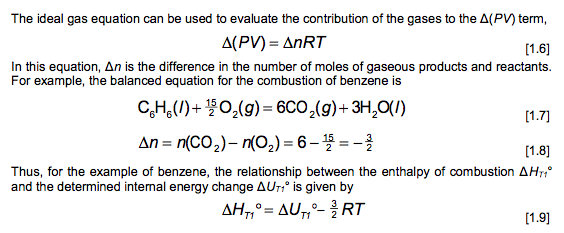
where Cr is the heat capacity (at constant volume) of the system. Since the temperature change is small, Cy can be considered constant, AUA = G(T2-T) [1.2] Then, since AUC = 0, AUT, = -(T2-T) [1.3] Equation 1.3 provides with the way to evaluate AUT1, if we can calculate Cr. This is straightforward when we know the heat capacity of the calorimeter. Products at T, . AU A U. T1 Reactants at T Products at T U = U (1.4) Finally, we can calculate the standard enthalpy of combustion from the standard internal energy change. From the definition of H, , = UT,+ (PV) [1.5] = T1 The ideal gas equation can be used to evaluate the contribution of the gases to the A(PV) term, A(PV)= ART (1.6) In this equation, An is the difference in the number of moles of gaseous products and reactants. For example, the balanced equation for the combustion of benzene is CHE(O)+ 10,(9) = 6CO2(9)+3H,0(1) [1.7] An= n(CO2) n(O2) = 6-=- [1.8] Thus, for the example of benzene, the relationship between the enthalpy of combustion AHTi and the determined internal energy change AUT is given by AH,, = AUT-RT (1.9) O T1 Assembly of data. At the completion of an experimental run, you should possess the following data: m: t: the net rise in temperature, in C. c1: volume of standard alkali solution used in acid titration, in ml. c2: mass of fuse wire consumed in firing, in g. mass of sample in g. Constants. You need to have recorded the following constants: N1: the normality of the alkali titrant. H1: the heat of combustion of the fuse wire in joules per gram. Qb: the heat of combustion of benzoic acid in joules per gram. Thermochemical corrections. Part of the measured change in temperature was generated by the formation of nitric acid in the bomb and by combustion of the fuse wire. This introduces errors which can be deducted from the calculations: e1: correction in joules for heat of formation of nitric acid. e2: correction in joules for heat of combustion of fuse wire. e1=(57.71J /ml of 1 N alkali) x c1 e2= c2 x H1 Energy equivalent of the calorimeter, W, determined from the benzoic acid standardization. Calculate this in joules per degree Celsius, using the following formula. mQ, + e1+e2 W t Gross heat of combustion, Q, for the test sample. Calculate this in joules per gram, using the following formula: tW -e1-e2 Q m AU and AH. Multiply Q by the molecular weight of the compound being analysed. Multiply this result by -1 to calculate AU (this is necessary to follow the modern thermodynamic sign convention. Calculate AHusing Equations 1.5 and 1.6. Discussion Questions 1. Why must the water in the bucket be weighed, when this weight of water appears nowhere in the calculation (or does it?) 2. The titration is carried out to correct for the formation of Nitric acid during the combustion reaction. What is the source of Nitrogen in the bomb and why does it react? 3.How important are the corrections, e1 and e2, to the accuracy of the overall measurement? 4.Discuss all valid sources of error in your report. Sample ID: Unknown C 0.9906 0.0063 Sample ID: Benzoic acid Mass of sample (in grams to 0.9856 four decimal places) Mass of wire consumed in 0.0107 firing: (in grams to four decimals places) Net rise in temperature C 2.6083 Titre volume of 0.079N 6.90 Na,Co, in mL to two decimal places 3.4079 10.48 Unknown C: M.W.=144.2 g/mol An= -3/2 where Cr is the heat capacity (at constant volume) of the system. Since the temperature change is small, Cy can be considered constant, AUA = G(T2-T) [1.2] Then, since AUC = 0, AUT, = -(T2-T) [1.3] Equation 1.3 provides with the way to evaluate AUT1, if we can calculate Cr. This is straightforward when we know the heat capacity of the calorimeter. Products at T, . AU A U. T1 Reactants at T Products at T U = U (1.4) Finally, we can calculate the standard enthalpy of combustion from the standard internal energy change. From the definition of H, , = UT,+ (PV) [1.5] = T1 The ideal gas equation can be used to evaluate the contribution of the gases to the A(PV) term, A(PV)= ART (1.6) In this equation, An is the difference in the number of moles of gaseous products and reactants. For example, the balanced equation for the combustion of benzene is CHE(O)+ 10,(9) = 6CO2(9)+3H,0(1) [1.7] An= n(CO2) n(O2) = 6-=- [1.8] Thus, for the example of benzene, the relationship between the enthalpy of combustion AHTi and the determined internal energy change AUT is given by AH,, = AUT-RT (1.9) O T1 Assembly of data. At the completion of an experimental run, you should possess the following data: m: t: the net rise in temperature, in C. c1: volume of standard alkali solution used in acid titration, in ml. c2: mass of fuse wire consumed in firing, in g. mass of sample in g. Constants. You need to have recorded the following constants: N1: the normality of the alkali titrant. H1: the heat of combustion of the fuse wire in joules per gram. Qb: the heat of combustion of benzoic acid in joules per gram. Thermochemical corrections. Part of the measured change in temperature was generated by the formation of nitric acid in the bomb and by combustion of the fuse wire. This introduces errors which can be deducted from the calculations: e1: correction in joules for heat of formation of nitric acid. e2: correction in joules for heat of combustion of fuse wire. e1=(57.71J /ml of 1 N alkali) x c1 e2= c2 x H1 Energy equivalent of the calorimeter, W, determined from the benzoic acid standardization. Calculate this in joules per degree Celsius, using the following formula. mQ, + e1+e2 W t Gross heat of combustion, Q, for the test sample. Calculate this in joules per gram, using the following formula: tW -e1-e2 Q m AU and AH. Multiply Q by the molecular weight of the compound being analysed. Multiply this result by -1 to calculate AU (this is necessary to follow the modern thermodynamic sign convention. Calculate AHusing Equations 1.5 and 1.6. Discussion Questions 1. Why must the water in the bucket be weighed, when this weight of water appears nowhere in the calculation (or does it?) 2. The titration is carried out to correct for the formation of Nitric acid during the combustion reaction. What is the source of Nitrogen in the bomb and why does it react? 3.How important are the corrections, e1 and e2, to the accuracy of the overall measurement? 4.Discuss all valid sources of error in your report. Sample ID: Unknown C 0.9906 0.0063 Sample ID: Benzoic acid Mass of sample (in grams to 0.9856 four decimal places) Mass of wire consumed in 0.0107 firing: (in grams to four decimals places) Net rise in temperature C 2.6083 Titre volume of 0.079N 6.90 Na,Co, in mL to two decimal places 3.4079 10.48 Unknown C: M.W.=144.2 g/mol An= -3/2