Answered step by step
Verified Expert Solution
Question
1 Approved Answer
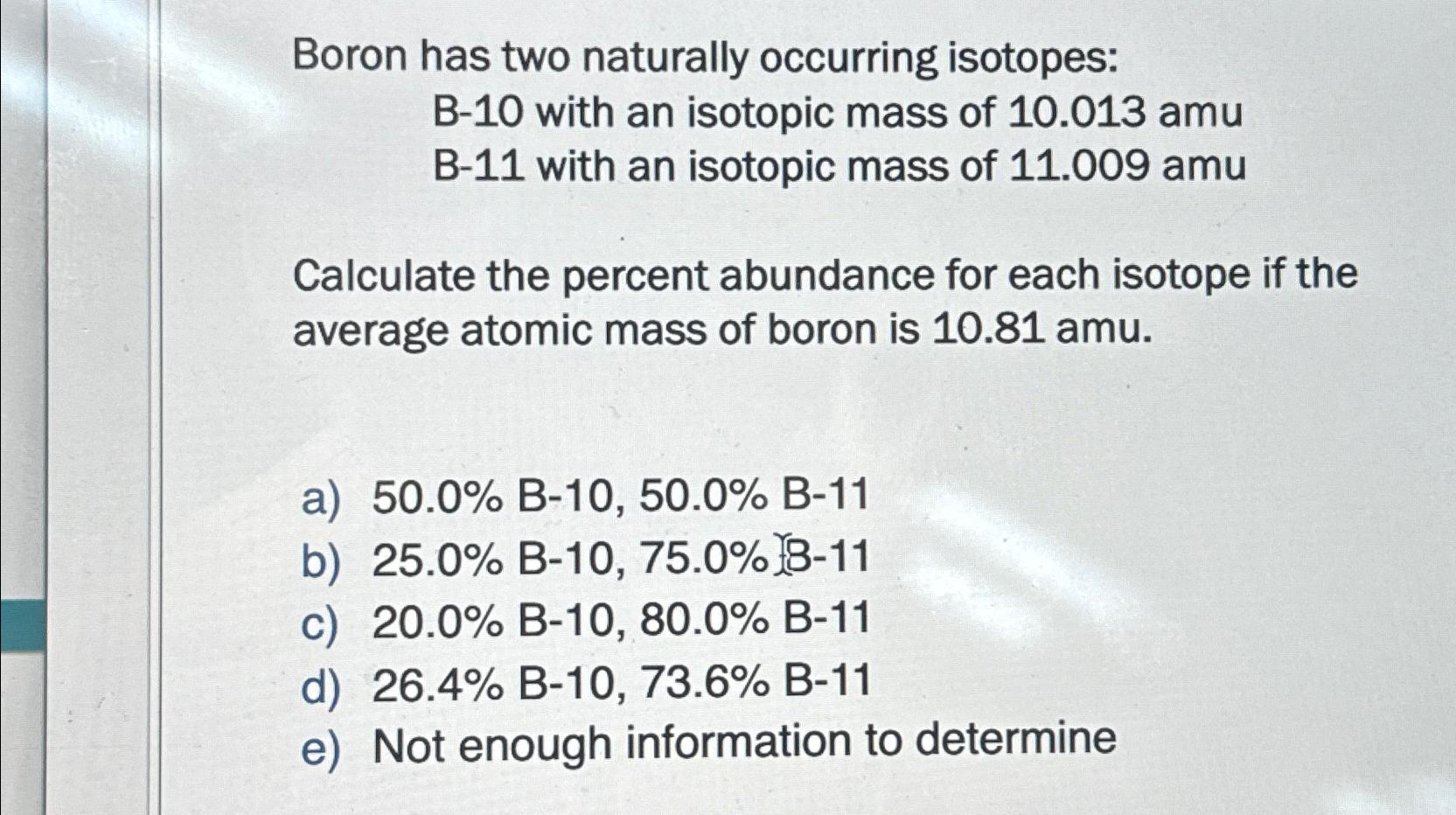
Boron has two naturally occurring isotopes: B-10 with an isotopic mass of 10.013amu B-11 with an isotopic mass of 11.009amu Calculate the percent
Boron has two naturally occurring isotopes:\ B-10 with an isotopic mass of
10.013a\\\\mu \ B-11 with an isotopic mass of
11.009a\\\\mu \ Calculate the percent abundance for each isotope if the average atomic mass of boron is
10.81a\\\\mu .\ a)
50.0%B-10,
50.0%B-11\ b)
25.0%B-10,75.0%\ c)
20.0%B-10,
80.0%B-11\ d)
26.4%B-10,73.6%B-11\ e) Not enough information to determine
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


