Answered step by step
Verified Expert Solution
Question
1 Approved Answer
both pls will give thumbs up! The combustion of 1.805g of fructose, C6H12O6(s), in a bomb calorimeter with a heat capacity of 5.20kJ/C results in
both pls will give thumbs up! 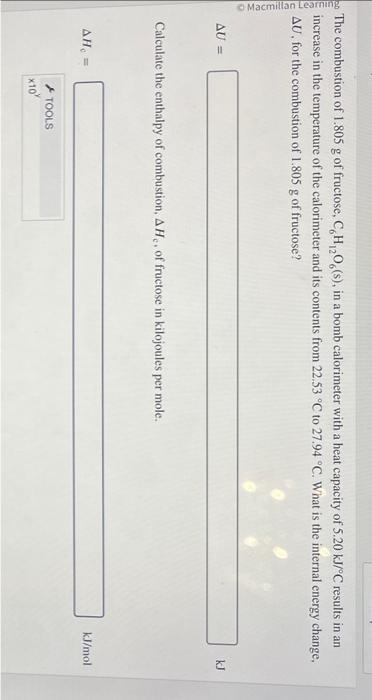

The combustion of 1.805g of fructose, C6H12O6(s), in a bomb calorimeter with a heat capacity of 5.20kJ/C results in an increase in the temperature of the calorimeter and its contents from 22.53C to 27.94C. What is the internal energy change, U, for the combustion of 1.805g of fructose? U= Calculate the enthalpy of combustion, Hc, of fructose in kilojoules per mole. Hc= /mol Suppose a boil water notice is sent out advising all residents in the area to boil their water before drinking it or using it for cooking. You need to boil 15.5 L of water using your natural gas (primarily methane, stove. What volume of natural gas is needed to boil the water if only 10.9% of the heat generated goes towards heating t ' water. Assume the density of methane is 0.668g/L, the density of water is 1.00g/mL, and that the water has an initial temperature of 22.1C. Enthalpy of formation values can be found in this table. Assume that gaseous water is formed in the combustion of methane 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


