Answered step by step
Verified Expert Solution
Question
1 Approved Answer
both questions Given the equilibrium reaction PCl5(g)PCl3(g)+Cl2(g) Calculate the number of moles of Cl2 produced at equilibrium when 1.00mol of PCl5 is heated at 246.9deg
both questions 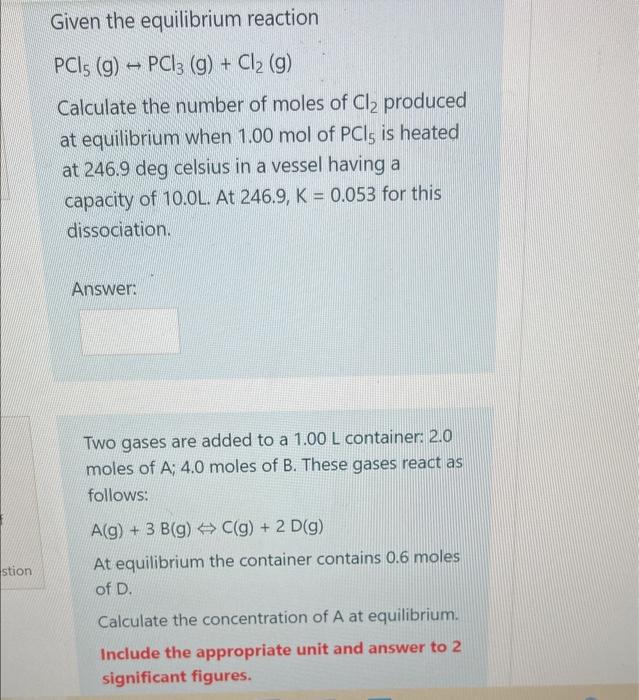 Given the equilibrium reaction PCl5(g)PCl3(g)+Cl2(g) Calculate the number of moles of Cl2 produced at equilibrium when 1.00mol of PCl5 is heated at 246.9deg celsius in a vessel having a capacity of 10.0L. At 246.9,K=0.053 for this dissociation. Answer: Two gases are added to a 1.00L container: 2.0 moles of A;4.0 moles of B. These gases react as follows: A(g)+3B(g)C(g)+2D(g) At equilibrium the container contains 0.6 moles of D. Calculate the concentration of A at equilibrium. Include the appropriate unit and answer to 2 significant figures
Given the equilibrium reaction PCl5(g)PCl3(g)+Cl2(g) Calculate the number of moles of Cl2 produced at equilibrium when 1.00mol of PCl5 is heated at 246.9deg celsius in a vessel having a capacity of 10.0L. At 246.9,K=0.053 for this dissociation. Answer: Two gases are added to a 1.00L container: 2.0 moles of A;4.0 moles of B. These gases react as follows: A(g)+3B(g)C(g)+2D(g) At equilibrium the container contains 0.6 moles of D. Calculate the concentration of A at equilibrium. Include the appropriate unit and answer to 2 significant figures
both questions 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


