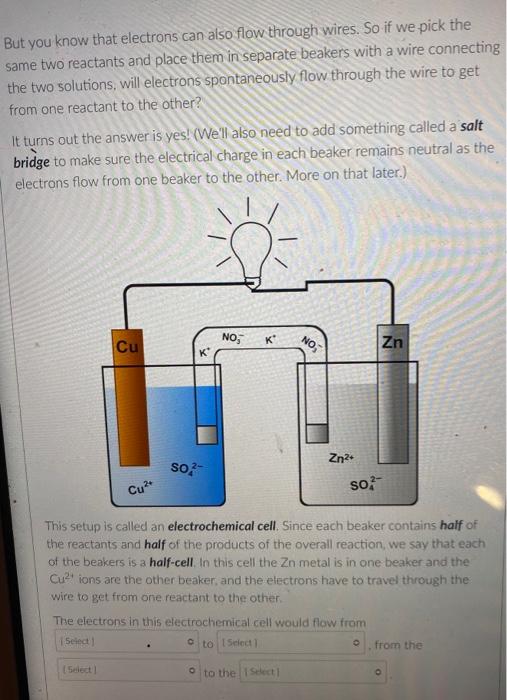
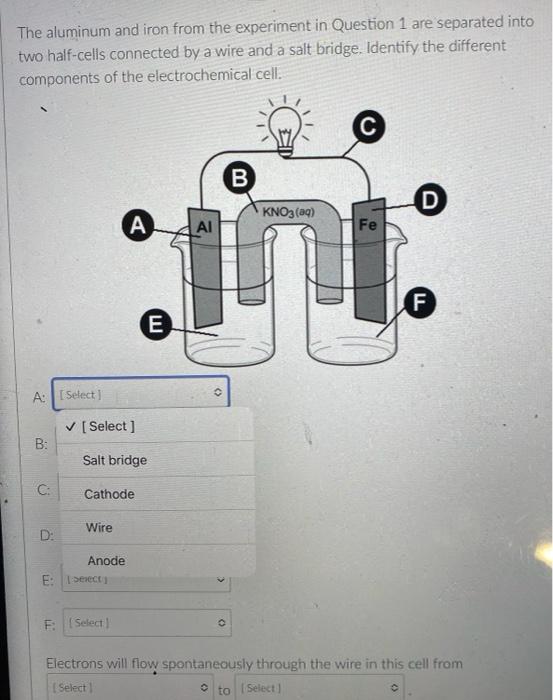
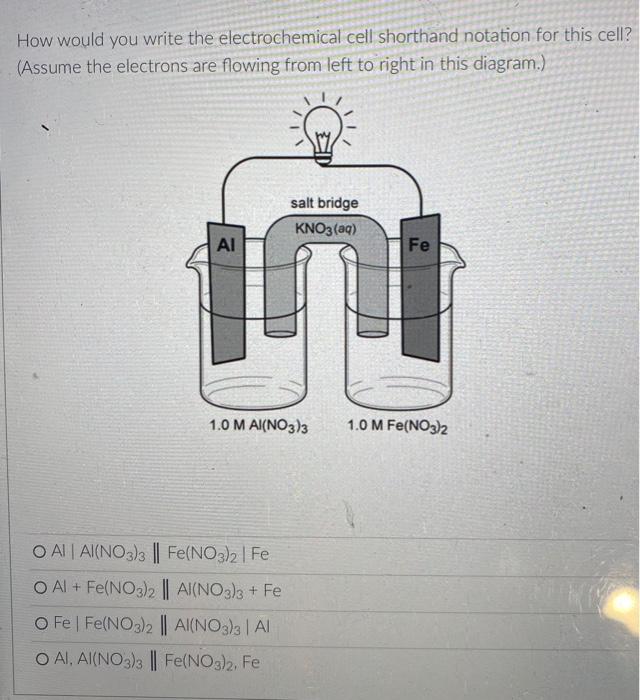
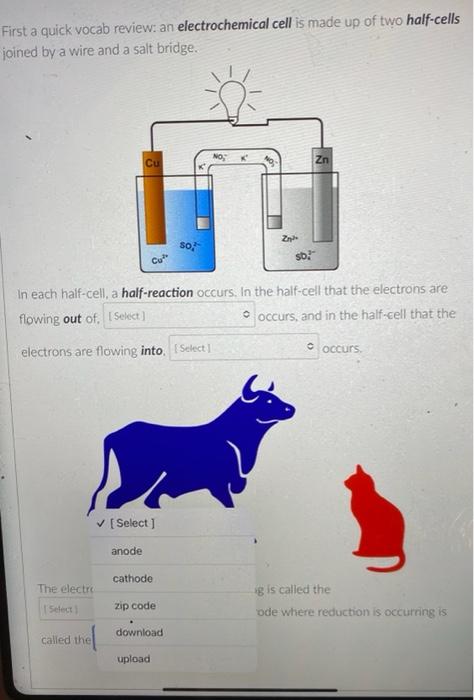
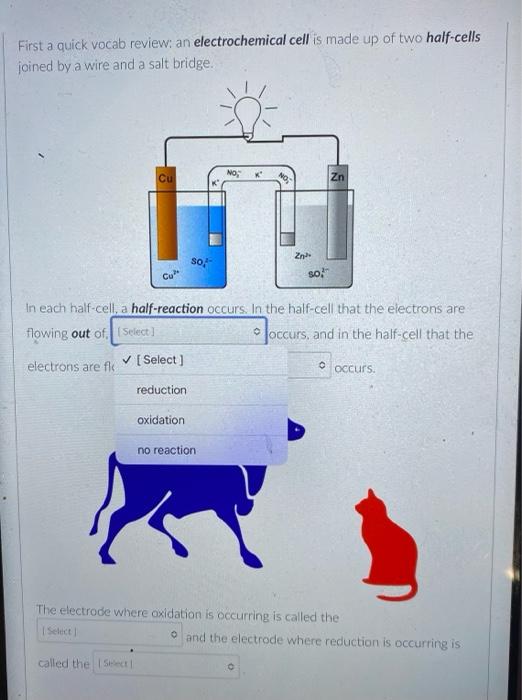
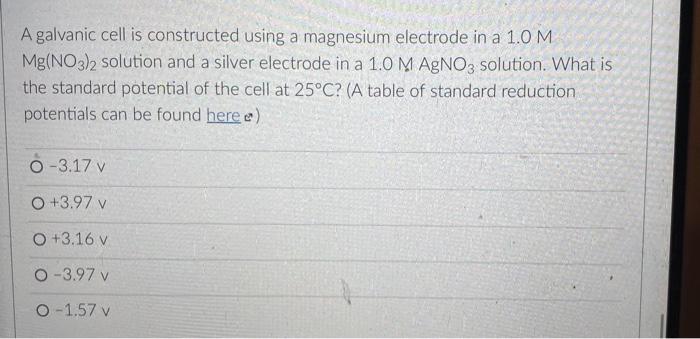
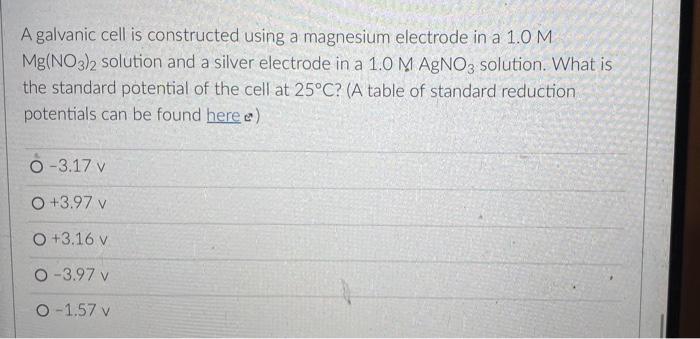
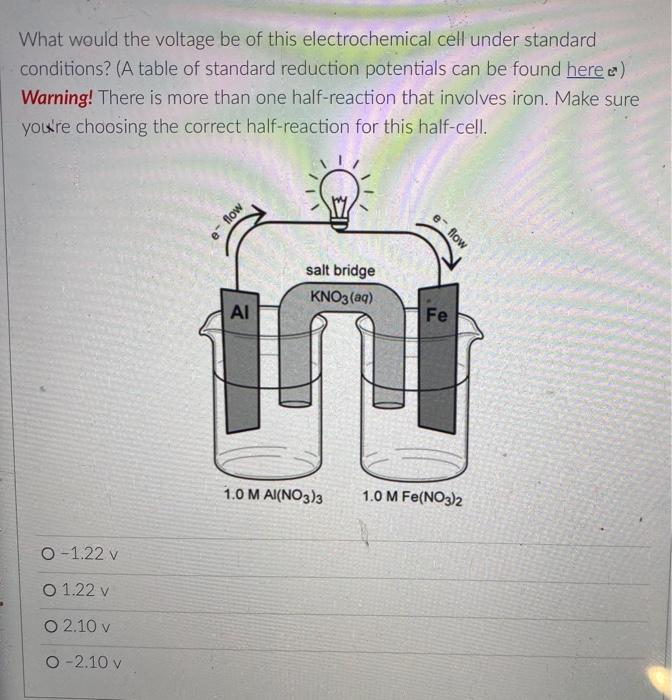
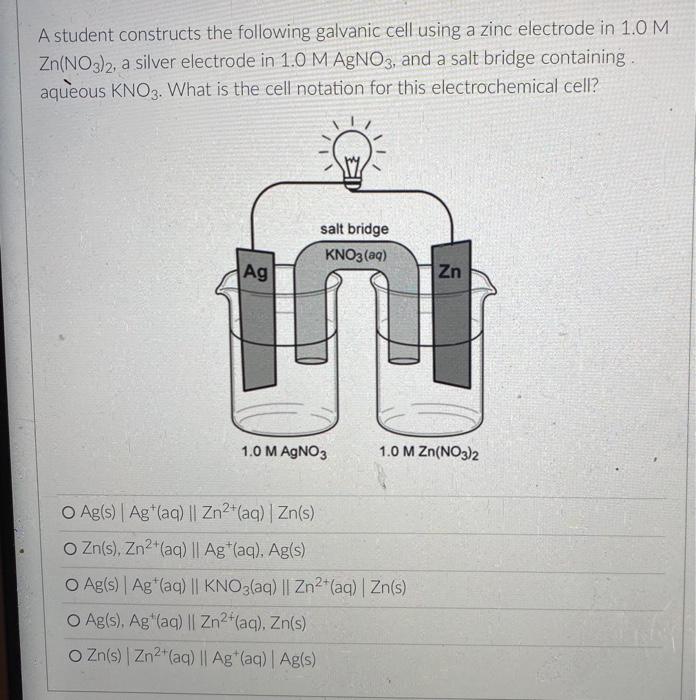
But you know that electrons can also flow through wires. So if we pick the same two reactants and place them in separate beakers with a wire connecting the two solutions, will electrons spontaneously flow through the wire to get from one reactant to the other? It turns out the answer is yes! (We'll also need to add something called a salt bridge to make sure the electrical charge in each beaker remains neutral as the electrons flow from one beaker to the other. More on that later.) NO, K Cu NO; Zn K Zn2 SO- Cu so, This setup is called an electrochemical cell. Since each beaker contains half of the reactants and half of the products of the overall reaction, we say that each of the beakers is a half-cell. In this cell the Zn metal is in one beaker and the Cu2!ions are the other beaker, and the electrons have to travel through the wire to get from one reactant to the other The electrons in this electrochemical cell would flow from Select o to Select from the Select o to the select A chemical reaction that produces electricity would be O spontaneous O non-spontaneous. When a piece of aluminum foil is dropped into an aqueous solution of FeCl2, the aluminum dissolves and solid iron is formed. 2Al(s) + 3Fe2+ (aq) 2A13+(aq) + 3Fe(s) You already know that in a spontaneous reaction the products have a lower free energy than the reactants do. In this reaction, where do the electrons have a lower free energy? O When they are in one of the solutions o When they are in the wire O When they are in the solid aluminum o When they are in the solid iron The aluminum and iron from the experiment in Question 1 are separated into two half-cells connected by a wire and a salt bridge. Identify the different components of the electrochemical cell. ( . D KNO3(aq) D A AI Fe F E > A: [Select) Select ] B: Salt bridge C: Cathode Wire D: Anode E: evet F Select Electrons will flow spontaneously through the wire in this cell from Select to Select How would you write the electrochemical cell shorthand notation for this cell? (Assume the electrons are flowing from left to right in this diagram.) salt bridge KNO3(aq) Fe 1.0 M Al(NO3)3 1.0 M Fe(NO3)2 O Al Al(NO3)3 || Fe(NO3)2 | Fe O Al + Fe(NO3)2 || Al(NO3)3 + Fe O Fe Fe(NO3)2 || Al(NO3)3 | Al O AI, AI(NO3)3 || Fe(NO3)2, Fe First a quick vocab review: an electrochemical cell is made up of two half-cells joined by a wire and a salt bridge. NO Cu Zn 50, Za sb Cu In each half-cell, a half-reaction occurs. In the half-cell that the electrons are flowing out of Select occurs, and in the half-cell that the electrons are flowing into. Select o occurs [Select ] anode cathode The electro ig is called the ode where reduction is occurring is Select zip code download called the upload First a quick vocab review: an electrochemical cell is made up of two half-cells joined by a wire and a salt bridge. Cu NO Zn SO Zn so Co In each half-cell, a half-reaction occurs. In the half-cell that the electrons are flowing out of. Select occurs, and in the half-cell that the electrons are fic [Select) reduction occurs oxidation no reaction The electrode where oxidation is occurring is called the Select and the electrode where reduction is occurring is called the sea In the activity series the half-reactions are written as oxidations and the lower free energy metals are at the bottom. In a table of standard reduction potentials, the half-reactions are written as reductions, and the metals with lower free energies are: O arranged alphabetically O scattered randomly among the list O still at the bottom O at the top A galvanic cell is constructed using a magnesium electrode in a 1.0 M Mg(NO3)2 solution and a silver electrode in a 1.0 M AgNO3 solution. What is the standard potential of the cell at 25C? (A table of standard reduction potentials can be found here) O-3.17 v O +3.97 v O +3.16 v O-3.97 v O -1.57 v A galvanic cell is constructed using a magnesium electrode in a 1.0 M Mg(NO3)2 solution and a silver electrode in a 1.0 M AgNO3 solution. What is the standard potential of the cell at 25C? (A table of standard reduction potentials can be found here) O-3.17 v O +3.97 v O +3.16 v O-3.97 v O -1.57 v What would the voltage be of this electrochemical cell under standard conditions? (A table of standard reduction potentials can be found here ) Warning! There is more than one half-reaction that involves iron. Make sure you're choosing the correct half-reaction for this half-cell. e- flow flow salt bridge KNO3(aq) AI Fe 1.0 M Al(NO3)3 1.0 M Fe(NO3)2 0 -1.22 v O 1.22 v O 2.10 v -2.10 v What would the voltage be of this electrochemical cell under standard conditions? Fe | Fe2+ || Pb4+, Pb2+ | Pt (A table of standard reduction potentials can be found here ) 0 -2.11 v 0 2.43 v 02.11 v O-2.43 v O 1.62 v For zero points but a chance to prove that you knew it, who is the final Disney Princess from the pause slides in the video lecture? (Yes I know technically she's not a Disney Princess, but I couldn't just leave her out completely.) O Scarlet Witch O Ursula O Ariel O Frozone Elsa Is this a galvanic cell or an electrolytic cell? What species is being produced at the cathode in this cell? Pt | H | H2 || Pb2+ | Pb Standard reduction potentials: 2H+ + 2e H2 E = 0.00 v Pb2+ + 2e Pb E = -0.0.13 v Electrolytic, H O Galvanic, Pb o Electrolytic, Pb O Galvanic, H2 A student constructs the following galvanic cell using a zinc electrode in 1.0 M Zn(NO3)2, a silver electrode in 1.0 M AgNO3, and a salt bridge containing aqueous KNO3. What is the cell notation for this electrochemical cell? w salt bridge KNO3(aq) Ag Zn 1.0 M AgNO3 1.0 MZn(NO3)2 O Ag(s) Ag+ (aq) || Zn2+(aq) | Zn(s) Zn(s), Zn2+(aq) || Ag (aq), Ag(s) O Ag(s) Ag (aq) || KNO3(aq) || Zn2+ (aq)| Zn(s) O Ag(s), Ag" (aq) || Zn2+(aq), Zn(s) o Zn(s) | Zn2+ (aq) || Ag (aq) | Ag(s)