Question
Butane gas is compressed from an initial state of 0.00687 m/mol, 0.4 MPa and 350K to a final state of 0.001225 m/mol, 2 MPa
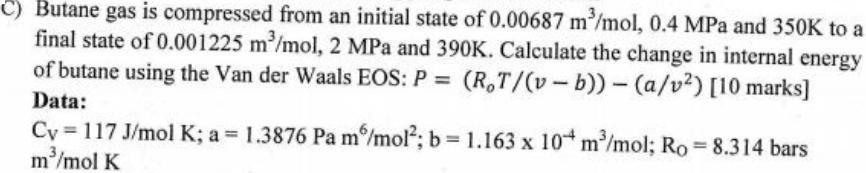
Butane gas is compressed from an initial state of 0.00687 m/mol, 0.4 MPa and 350K to a final state of 0.001225 m/mol, 2 MPa and 390K. Calculate the change in internal energy of butane using the Van der Waals EOS: P = (R,T/(v- b)) (a/v?) [10 marks] Data: Cy =117 J/mol K; a = 1.3876 Pa m/mol; b= 1.163 x 10* m/mol; Ro 8.314 bars m/mol K
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Chemical Engineering Thermodynamics
Authors: J. M. Smith, H. C. Van Ness, M. M. Abbott
7th edition
71247084, 978-0071247085
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



