Answered step by step
Verified Expert Solution
Question
1 Approved Answer
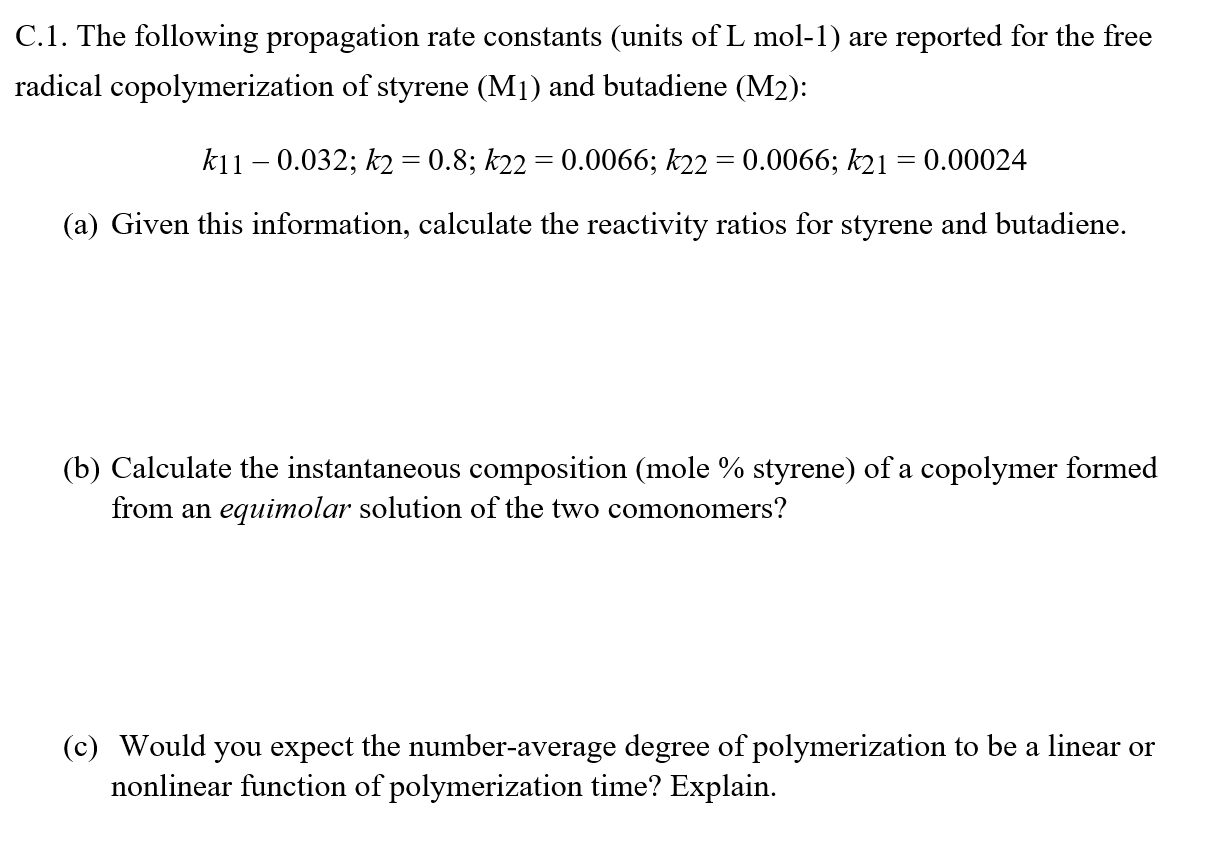
C . 1 . The following propagation rate constants ( units of Lmol - 1 ) are reported for the free radical copolymerization of styrene
C The following propagation rate constants units of Lmol are reported for the free
radical copolymerization of styrene and butadiene :
;;;;
a Given this information, calculate the reactivity ratios for styrene and butadiene.
b Calculate the instantaneous composition mole styrene of a copolymer formed
from an equimolar solution of the two comonomers?
c Would you expect the numberaverage degree of polymerization to be a linear or
nonlinear function of polymerization time? Explain.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


