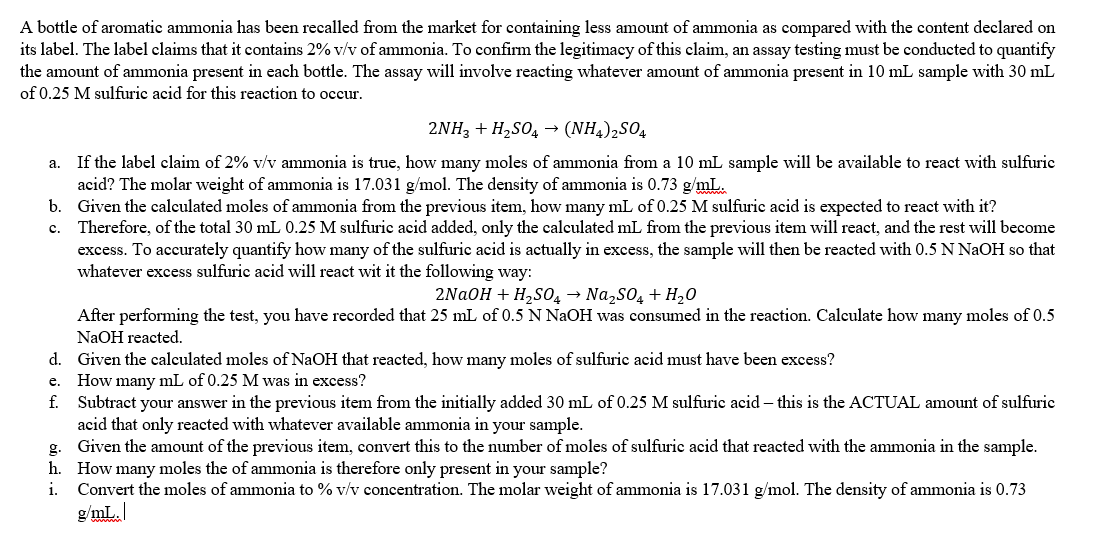
c. A bottle of aromatic ammonia has been recalled from the market for containing less amount of ammonia as compared with the content declared on its label. The label claims that it contains 2%v/v of ammonia. To confirm the legitimacy of this claim, an assay testing must be conducted to quantify the amount of ammonia present in each bottle. The assay will involve reacting whatever amount of ammonia present in 10 mL sample with 30 mL of 0.25 M sulfuric acid for this reaction to occur. 2NH3 + H2SO4 (NH4)2S04 a. If the label claim of 2% v/v ammonia is true, how many moles of ammonia from a 10 mL sample will be available to react with sulfuric acid? The molar weight of ammonia is 17.031 g/mol. The density of ammonia is 0.73 g/mL. b. Given the calculated moles of ammonia from the previous item, how many mL of 0.25 M sulfuric acid is expected to react with it? Therefore, of the total 30 mL 0.25 M sulfuric acid added, only the calculated mL from the previous item will react, and the rest will become excess. To accurately quantify how many of the sulfuric acid is actually in excess, the sample will then be reacted with 0.5 N NaOH so that whatever excess sulfuric acid will react wit it the following way: 2NaOH + H2SO4 Na2SO4 + H2O After performing the test, you have recorded that 25 mL of 0.5 N NaOH was consumed in the reaction. Calculate how many moles of 0.5 NaOH reacted. d. Given the calculated moles of NaOH that reacted, how many moles of sulfuric acid must have been excess? How many mL of 0.25 M was in excess? f. Subtract your answer in the previous item from the initially added 30 mL of 0.25 M sulfuric acid - this is the ACTUAL amount of sulfuric acid that only reacted with whatever available ammonia in your sample. g. Given the amount of the previous item, convert this to the number of moles of sulfuric acid that reacted with the ammonia in the sample. h. How many moles the of ammonia is therefore only present in your sample? i. Convert the moles of ammonia to % v/v concentration. The molar weight of ammonia is 17.031 g/mol. The density of ammonia is 0.73 e. g/mL. /







