Question
C) A fuel-air mixture enters a closed cylinder of an internal combustion engine at 0.10 MPa and 25C. The mixture is then adiabatically and
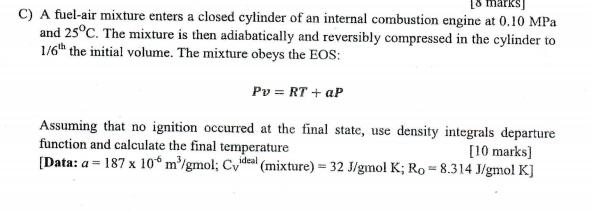
C) A fuel-air mixture enters a closed cylinder of an internal combustion engine at 0.10 MPa and 25C. The mixture is then adiabatically and reversibly compressed in the cylinder to 1/6h the initial volume. The mixture obeys the EOS: Pv = RT + aP Assuming that no ignition occurred at the final state, use density integrals departure function and calculate the final temperature [Data: a = 187 x 10 m'/gmol; Cvdeal (mixture) = 32 J/gmol K; Ro = 8.314 J/gmol K] [10 marks]
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
given initial pressure 01MPa initial temperature 25c 298 k After compressing adiabatical...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics An Interactive Approach
Authors: Subrata Bhattacharjee
1st edition
130351172, 978-0130351173
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



