Question
Table Q2 (C) below gives some property values for water at 120C and 1.985 bars. Use Table Q2 (C) and with the aid of
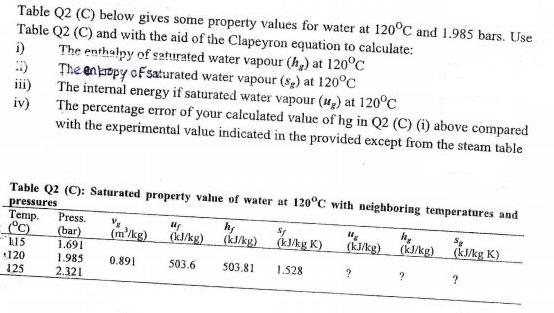
Table Q2 (C) below gives some property values for water at 120C and 1.985 bars. Use Table Q2 (C) and with the aid of the Clapeyron equation to calculate: i) The enthalpy of seturated water vapour (h,) at 120C :) The enkrtpy of saturated water vapour (sp) at 120C iii) The internal energy if saturated water vapour (u,) at 120C iv) The percentage error of your calculated value of hg in Q2 (C) (i) above compared with the experimental value indicated in the provided except from the steam table Table Q2 (C): Saturated property value of water at 120C with neighboring temperatures and pressures Temp. C) Press. (bar) 1.691 (m'/kg) (k/kg) ) (kJkg) (kukg) kJAg K). (k/kg) (kkg K) (kJ/kg K) L15 120 125 1.985 0.891 503.6 503.81 1.528 2.321
Step by Step Solution
3.56 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Complex Variables and Applications
Authors: James Brown, Ruel Churchill
8th edition
73051942, 978-0073051949
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



