Answered step by step
Verified Expert Solution
Question
1 Approved Answer
c) A mixture of four compounds as shown in Figure 1 was analyzed using supercritical fluid chromatography (SFC) with carbon dioxide as the supercritical fluid
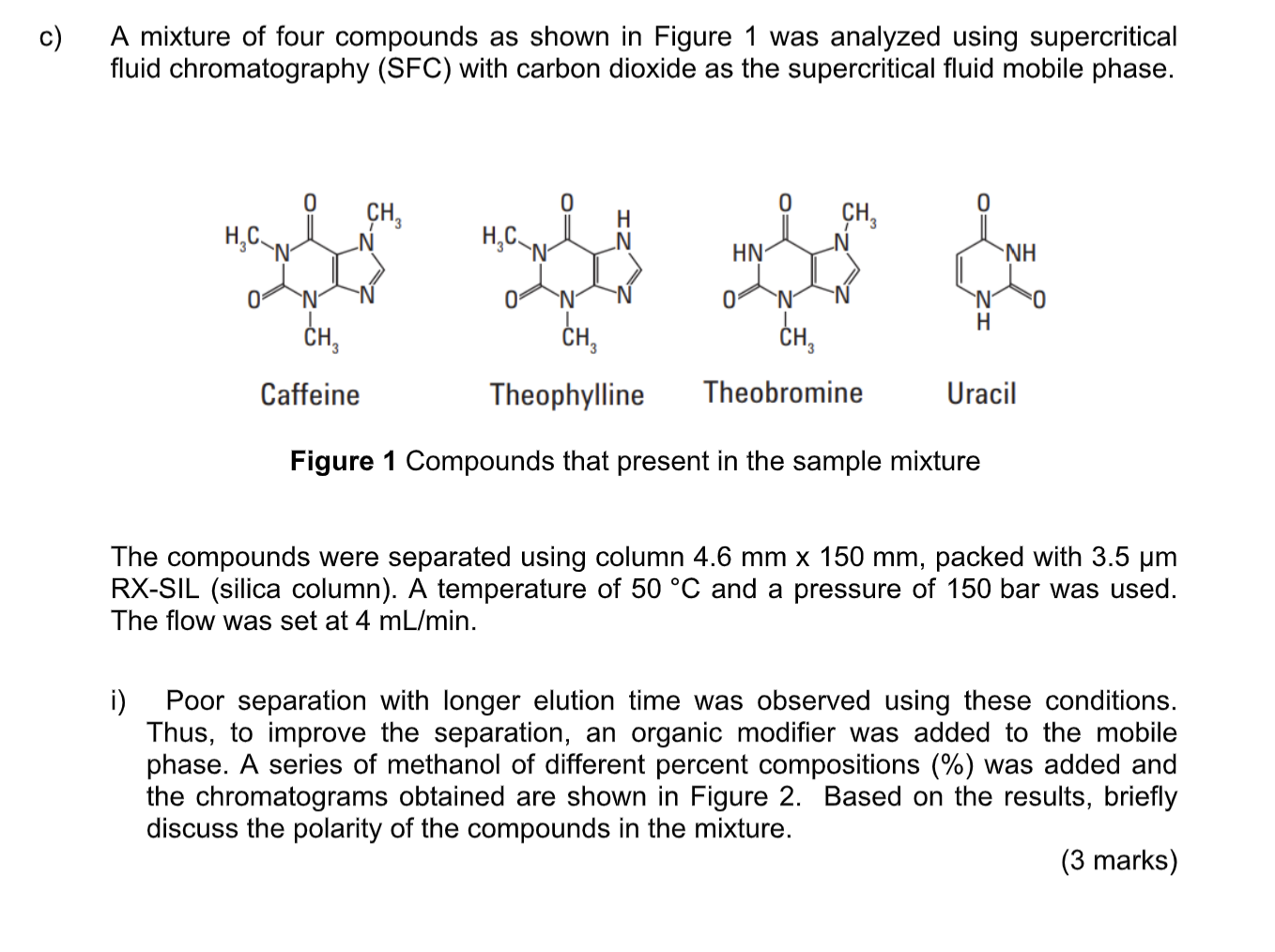
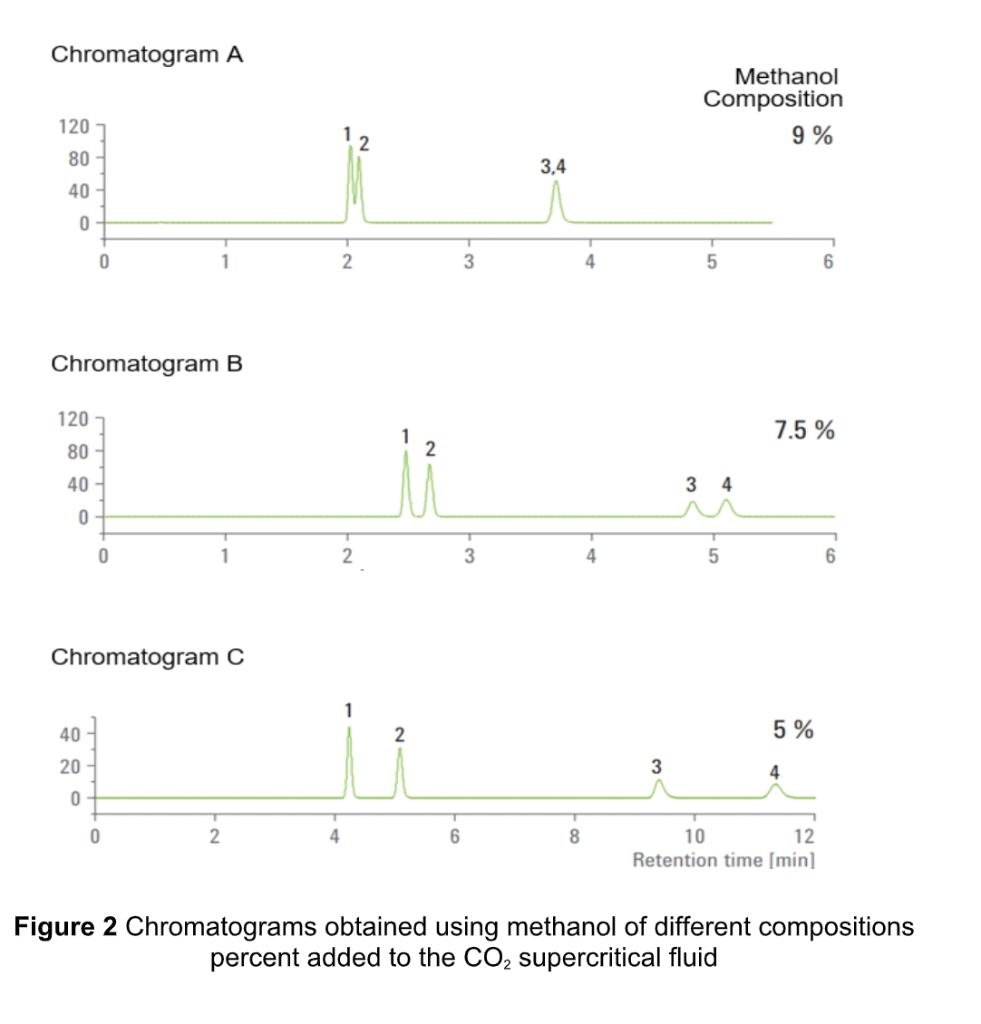

c) A mixture of four compounds as shown in Figure 1 was analyzed using supercritical fluid chromatography (SFC) with carbon dioxide as the supercritical fluid mobile phase. 0 0 CH CH .. .. HN " NH 0 N 0 0 0 H CH, CH CH, Caffeine Theophylline Theobromine Uracil Figure 1 Compounds that present in the sample mixture 1 The compounds were separated using column 4.6 mm x 150 mm, packed with 3.5 um RX-SIL (silica column). A temperature of 50 C and a pressure of 150 bar was used. The flow was set at 4 mL/min. i) Poor separation with longer elution time was observed using these conditions. Thus, to improve the separation, an organic modifier was added to the mobile phase. A series of methanol of different percent compositions (%) was added and the chromatograms obtained are shown in Figure 2. Based on the results, briefly discuss the polarity of the compounds in the mixture. (3 marks) Chromatogram A Methanol Composition 9% 120 12 80 3.4 40 0 0 2 3 4 5 6 Chromatogram B 120 1 7.5 % 80 2. 3 4 40 0 0 2 3 5 6 Chromatogram C 40 2 5 % 20 3 4 0 0 6 8 10 12 Retention time [min] Figure 2 Chromatograms obtained using methanol of different compositions percent added to the CO, supercritical fluid ii) Explain why methanol was used to improve the separation. (3 marks) iii) State a parameter that can be used to optimise the above separation (to shorten the analysis time without demolishing the resolution). Explain how it works. (3 marks) iv) Based on chromatograms in Figure 2, which condition of organic modifier percentage (%) should be chosen to start-off for optimising the separation. (2 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


