Answered step by step
Verified Expert Solution
Question
1 Approved Answer
c and d and e please Problem 2. A chemical constituent flows between three well-mixed tanks, also called reactors, as depicted in Figure below. Steady-state
c and d and e please 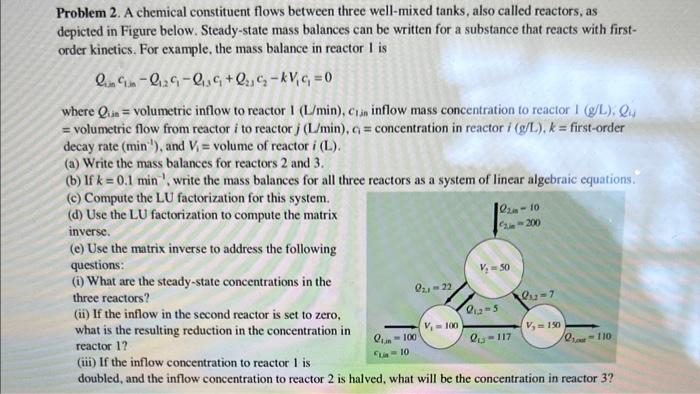
Problem 2. A chemical constituent flows between three well-mixed tanks, also called reactors, as depicted in Figure below. Steady-state mass balances can be written for a substance that reacts with firstorder kinetics. For example, the mass balance in reactor 1 is Q1,nc1,inQ1,2c1Q13c1+Q2,1c2kV1c1=0 where Qin= volumetric inflow to reactor 1 ( L/min),c1in inflow mass concentration to reactor 1(g/L),Qis = volumetric flow from reactor i to reactor j(L/min),c1= concentration in reactor i(g/L),k= first-order decay rate (min1), and V1= volume of reactor i(L). (a) Write the mass balances for reactors 2 and 3 . (b) If k=0.1min1, write the mass balances for all three reactors as a system of linear algebraic equations. (c) Compute the LU factorization for this system. (d) Use the LU factorization to compute the matrix inverse. (c) Use the matrix inverse to address the following questions: (i) What are the steady-state concentrations in the three reactors? (ii) If the inflow in the second reactor is set to zero, what is the resulting reduction in the concentration in reactor 1 ? (iii) If the inflow concentration to reactor 1 is doubled, and the inflow concentration to reactor 2 is halved, what will be the concentration in reactor 3 ? Problem 2. A chemical constituent flows between three well-mixed tanks, also called reactors, as depicted in Figure below. Steady-state mass balances can be written for a substance that reacts with firstorder kinetics. For example, the mass balance in reactor 1 is Q1,nc1,inQ1,2c1Q13c1+Q2,1c2kV1c1=0 where Qin= volumetric inflow to reactor 1 ( L/min),c1in inflow mass concentration to reactor 1(g/L),Qis = volumetric flow from reactor i to reactor j(L/min),c1= concentration in reactor i(g/L),k= first-order decay rate (min1), and V1= volume of reactor i(L). (a) Write the mass balances for reactors 2 and 3 . (b) If k=0.1min1, write the mass balances for all three reactors as a system of linear algebraic equations. (c) Compute the LU factorization for this system. (d) Use the LU factorization to compute the matrix inverse. (c) Use the matrix inverse to address the following questions: (i) What are the steady-state concentrations in the three reactors? (ii) If the inflow in the second reactor is set to zero, what is the resulting reduction in the concentration in reactor 1 ? (iii) If the inflow concentration to reactor 1 is doubled, and the inflow concentration to reactor 2 is halved, what will be the concentration in reactor 3 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


