Answered step by step
Verified Expert Solution
Question
1 Approved Answer
c) Consider a cylindrical vessel of total volume 0.01m3, which contains 0.001m of water; the remainder of the vessel is filled with initially dry air.
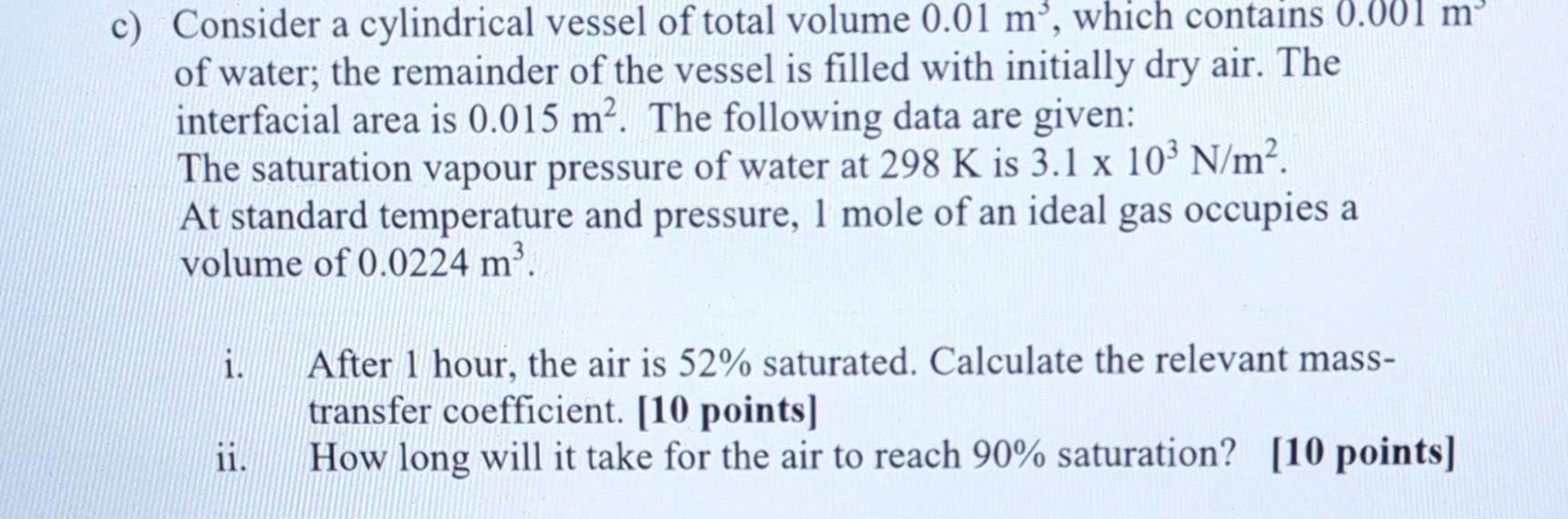
c) Consider a cylindrical vessel of total volume 0.01m3, which contains 0.001m of water; the remainder of the vessel is filled with initially dry air. The interfacial area is 0.015m2. The following data are given: The saturation vapour pressure of water at 298K is 3.1103N/m2. At standard temperature and pressure, 1 mole of an ideal gas occupies a volume of 0.0224m3. i. After 1 hour, the air is 52% saturated. Calculate the relevant masstransfer coefficient. [10 points] ii. How long will it take for the air to reach 90% saturation? [10 points]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


