Question: C. Cycloatiknnes Compare the last three compounds in the table above. Which property is identical and which properties are different? Why? Goals - Build molecular
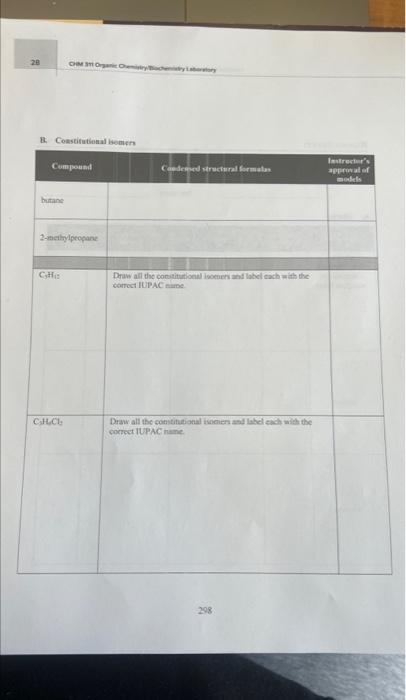
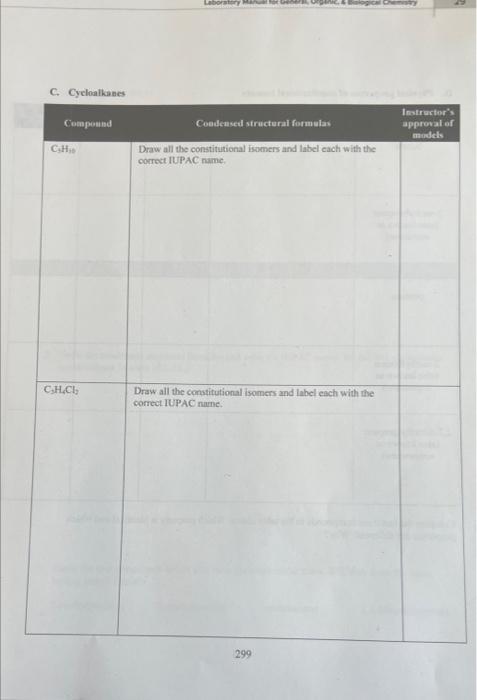
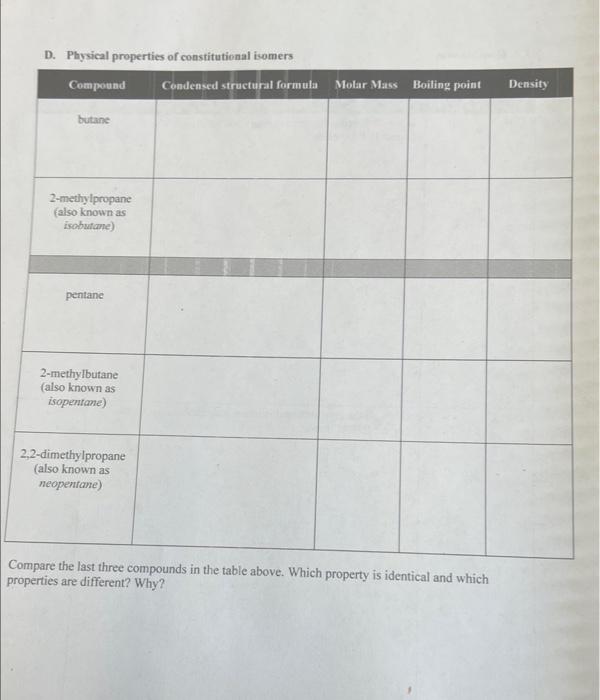

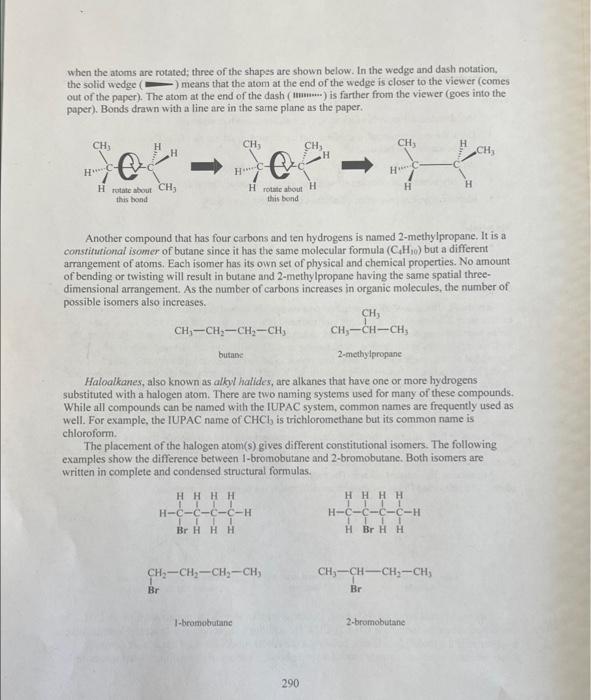



C. Cycloatiknnes Compare the last three compounds in the table above. Which property is identical and which properties are different? Why? Goals - Build molecular models of alkanes, cycloalkanes, and haloalkanes. - Draw three-dimensional, complete, and condensed structural formulas for alkanes, cycloalkanes, and haloalkanes. - Write IUPAC names for alkanes, cycloalkanes, and haloalkanes. - Identify isomers of compounds with the same molecular formulas. - Identify physical properties of alkanes using a reference book. Materials - Molecular model kits - Merek Index or CRC Handbook of Chemistry and Physics Discussion The basic structure of all organic compounds centers around the electron arrangement of the carbon atom. Carbon has four valence electrons and it always obeys the octet rule. Thus, it always forms four covalent bonds. VSEPR theory indicates that when carbon makes four single bonds, the molecular geometry is tetrahedral. A molecular model kit will have the carbon bonds in this: tetrahedral arrangement and will allow four bonds to be formed around the carbon. Similarly, the model kit will allow hydrogen to form one bond. When the hydrogens and carbons are put together to form the desired molecule, the resulting model will show the shape by deseribing the spatial arrangement of the atoms. The simplest organic family known as alkanes includes saturated hydrocarbons, those containing only carbon and hydrogen atoms with all single bonds. It is useful to make models of compounds to help visualize the actual three-dimensional shape of a molecule from its written twodimensional formula. The chemical and physical properties of a compound are dependent upon the three-dimensional shape of the molecule. Methane is the simplest alkane. The molecule contains one carbon atom and four hydrogen atoms. The following formulas show three different ways to tepresent methane. A more complex molecule is butane. Butane is an alkane with the molecular formula C4H10. Its condensed structural formula is CH3CH2CH2CH3. Although the condensed structural formula makes it appear as if the molecule is linear, building a molecular model shows it has a more complex shape. Because atoms may rotate around a single bond, a variety of three-dimensional shapes result 289 when the atoms are rotated; three of the shapes are shown below. In the wedge and dash notation, the solid wedge ( ) means that the atom at the end of the wedge is closer to the viewer (comes. out of the paper). The atom at the end of the dash ( musuw.) is farther from the viewer (goes into the paper). Bonds drawn with a line are in the same plane as the paper. Another compound that has four carbons and ten hydrogens is named 2 -methylpropane. It is a constitutional isomer of butane since it has the same molecular formula (C4H10) but a different arrangement of atoms. Each isomer has its own set of physical and chemical properties. No amount of bending or twisting will result in butane and 2 -methylpropane having the same spatial threedimensional arrangement. As the number of carbons increases in organic molecules, the number of possible isomers also increases. CH3CH2CH2CH3 butane 2 -methylpropane Haloalkanes, also known as alkyl halides, are alkanes that have one or more hydrogens substituted with a halogen atom. There are two naming systems used for many of these compounds. While all compounds can be named with the IUPAC system, common names are frequently used as well. For example, the IUPAC name of CHCl3 is trichloromethane but its common name is chloroform. The placement of the halogen atom(s) gives different constitutional isomers. The following examples show the difference between I-bromobutane and 2-bromobutane. Both isomers are written in complete and condensed structural formulas. Cycloalkanes have a ring structure in the molecule. The formation of the ring requires the loss of two hydrogens. For example, the molecular formula for butane is C4H10 but the molecular formula for cyclobutane is C4H8. The complete structural formula, condensed structural formula, and skeletal structure for cyclobutane are shown in the following example. It is important to remember that in a skeletal structure, all the carbon atoms and all the hydrogen atoms that are bound to carbon atoms are omitted. It is understood that a carbon atom is present at every point where two lines meet. Thus, each comer represents carbon with enough hydrogens attached to provide the carbon atom with four bonds. A. Alkanes 1. Use molecular model kits to build the models for methane, ethane and propane. Have the instructor approve the models. 2. For methane, draw the three-dimensional tetrahedral formula. The "wedge" represents a bond coming out of the page and the "dash" represents the bond going into the page. Solid lines represent bonds in the plane of the page. 3. Draw the complete structural formula, condensed structural formula, and molecular formula for each model on the report sheet. B. Constitutional isomers 1. Build the models for butane and 2 -methyl propane. Notice that the models cannot be twisted or turned to become the same molecule. Demonstrate to the instructor how the butane model must be changed in order to make a 2-methylpropane model. Have the instructor approve the models. 2. Make the three constitutional isomers with the molecular formula C5H12. Draw their condensed structural formulas and write the IUPAC name for each structure. Have the instructor approve the models and check the isomers. 3. Make the four constitutional isomers with the molecular formula C3H6Cl2. Draw their condensed structural formulas and write the IUPAC name for each structure. Have the instructor approve the models and check the isomers. C. Cycloalkanes 1. Make the five constitutional isomers with the molecular formula C3H10. Note: because these are cycloalkanes, each one must contain a ring of carbon atoms. Draw their condensed structural formulas and write the IUPAC name for each structure. Have the instructor approve the models and check the isomers. 2. Make both of the constitutional isomers with the molecular formula C3H4Cl2. Note: because these are cycloalkanes, each one must contain a ring of carbon atoms. Draw their condensed structural formulas and write the IUPAC name for each structure. Have the instructor approve the models and check the isomers. 3. Return all of the model pieces to the correct containers. D. Physical properties of constitutional isomers 1. Draw the condensed structural formulas for the compounds listed on the report sheet. 2. Following the instructor's directions, use a chemistry handbook to look up the physical properties for each compound and record the information on the report sheet. Include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


