Answered step by step
Verified Expert Solution
Question
1 Approved Answer
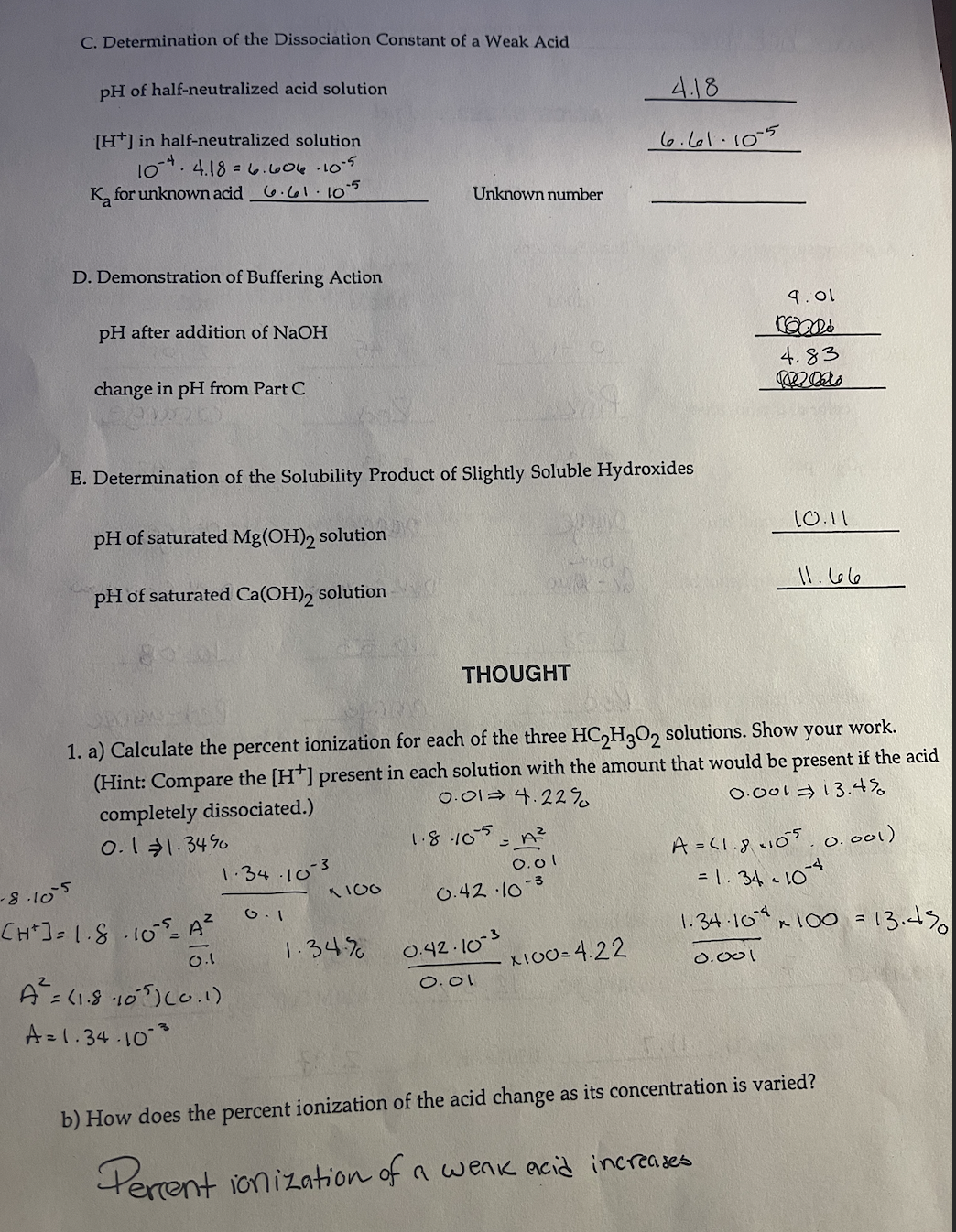
C . Determination of the Dissociation Constant of a Weak Acid p H of half - neutralized acid solution 4 . 1 8 H +
C Determination of the Dissociation Constant of a Weak Acid
of halfneutralized acid solution
in halfneutralized solution
for unknown acid
Unknown number
D Demonstration of Buffering Action
after addition of NaOH
change in from Part
of saturated solution
of saturated solution
E Determination of the Solubility Product of Slightly Soluble Hydroxides
THOUGHT
a Calculate the percent ionization for each of the three solutions. Show your work.
Hint: Compare the present in each solution with the amount that would be present if the acid
completely dissociated.
b How does the percent ionization of the acid change as its concentration is varied?
Percent ionization of a wenk acid increases
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


