Answered step by step
Verified Expert Solution
Question
1 Approved Answer
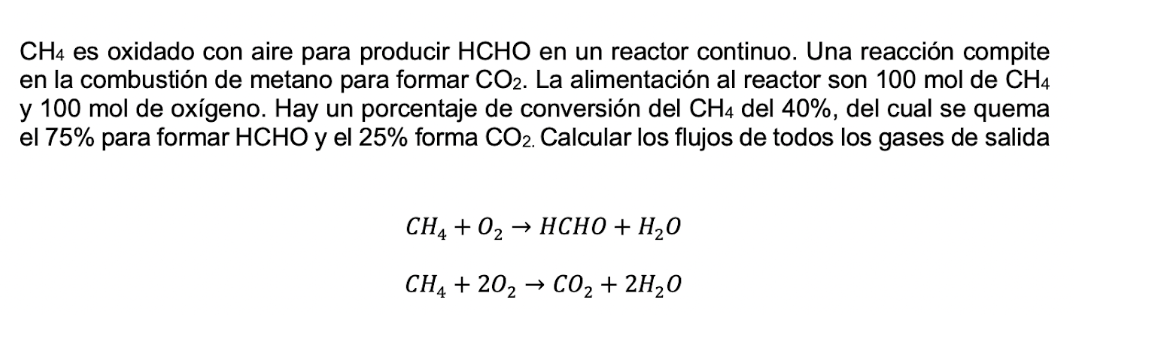
C H 4 es oxidado con aire para producir HCHO en un reactor continuo. Una reacci n compite en la combusti n de metano para
es oxidado con aire para producir HCHO en un reactor continuo. Una reaccin compite
en la combustin de metano para formar La alimentacin al reactor son mol de
y mol de oxgeno Hay un porcentaje de conversin del del del cual se quema
el para formar HCHO y el forma Calcular los flujos de todos los gases de salida
HCHO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


