Answered step by step
Verified Expert Solution
Question
1 Approved Answer
c) i) Explain the origin of the volcano dependence of the ORR activity on the binding energy of the oxygen intermediate on the catalyst,
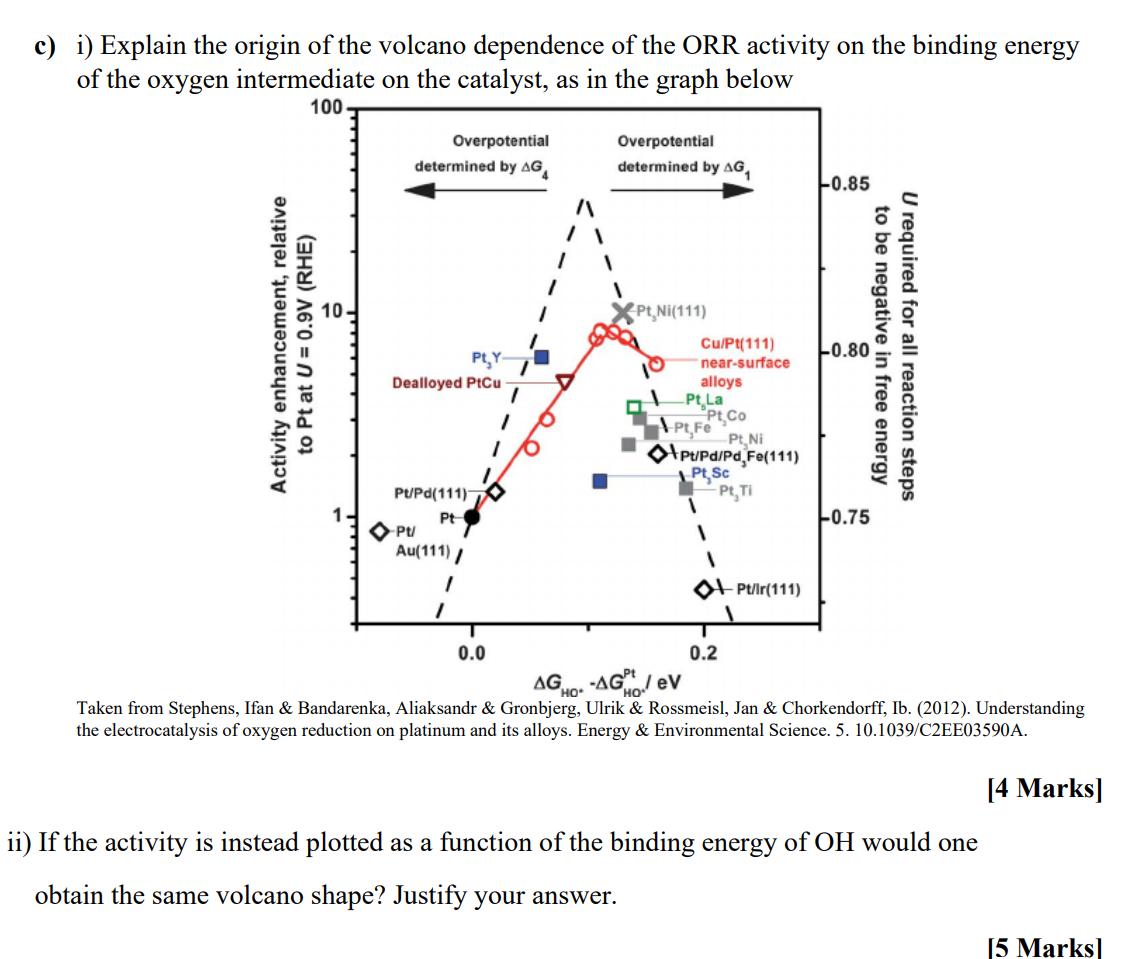
c) i) Explain the origin of the volcano dependence of the ORR activity on the binding energy of the oxygen intermediate on the catalyst, as in the graph below Activity enhancement, relative to Pt at U = 0.9V (RHE) 100 Overpotential determined by AG Overpotential determined by AG -0.85 PLY Dealloyed PtCu Pt, Ni(111) Cu/Pt(111) near-surface alloys Pt La PU/Pd(111) Pt- Pt/ Au(111)/ 0.0 Pt Co Pt,Fe Pt Ni Pt/Pd/Pd Fe(111) PtSc Pt, Ti 0.2 Pt/Ir(111) -0.80 -0.75 to be negative in free energy U required for all reaction steps AGHO--AG ev Taken from Stephens, Ifan & Bandarenka, Aliaksandr & Gronbjerg, Ulrik & Rossmeisl, Jan & Chorkendorff, Ib. (2012). Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy & Environmental Science. 5. 10.1039/C2EE03590A. ii) If the activity is instead plotted as a function of the binding energy of OH would one obtain the same volcano shape? Justify your answer. [4 Marks] [5 Marks]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The volcano plot shown in the image demonstrates the relationship between the electrocatalytic activity for the oxygen reduction reaction ORR and the binding energy of the oxygen intermediate on vario...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


