Answered step by step
Verified Expert Solution
Question
1 Approved Answer
c) In the conventional methanol production, methanol is produced from petroleum product (synthesis gas) via hydrogenation of CO. CO+ 2H2 CH3OH 400 mol/s H2 and
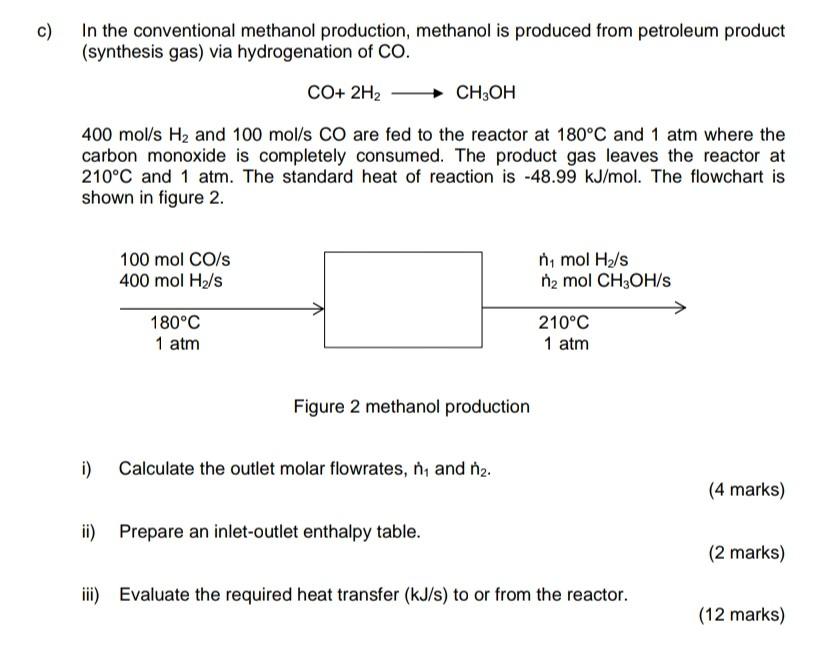
c) In the conventional methanol production, methanol is produced from petroleum product (synthesis gas) via hydrogenation of CO. CO+ 2H2 CH3OH 400 mol/s H2 and 100 mol/s CO are fed to the reactor at 180C and 1 atm where the carbon monoxide is completely consumed. The product gas leaves the reactor at 210C and 1 atm. The standard heat of reaction is -48.99 kJ/mol. The flowchart is shown in figure 2. 100 mol CO/s 400 mol He/s ni mol H2/s n2 mol CH3OH/S 180C 1 atm 210C 1 atm Figure 2 methanol production i) Calculate the outlet molar flowrates, ni and nz. (4 marks) ii) Prepare an inlet-outlet enthalpy table. (2 marks) iii) Evaluate the required heat transfer (kJ/s) to or from the reactor. (12 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


