Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calcium carbide (CaC2) is produced when CaO, known as quicklime, reacts with carbon at high temperature. CaO(s)+3C(s)CaC2(s)+CO(g)rH=+445kJ/mol If 10.9g of carbon (12.01g/mol) are allowed to
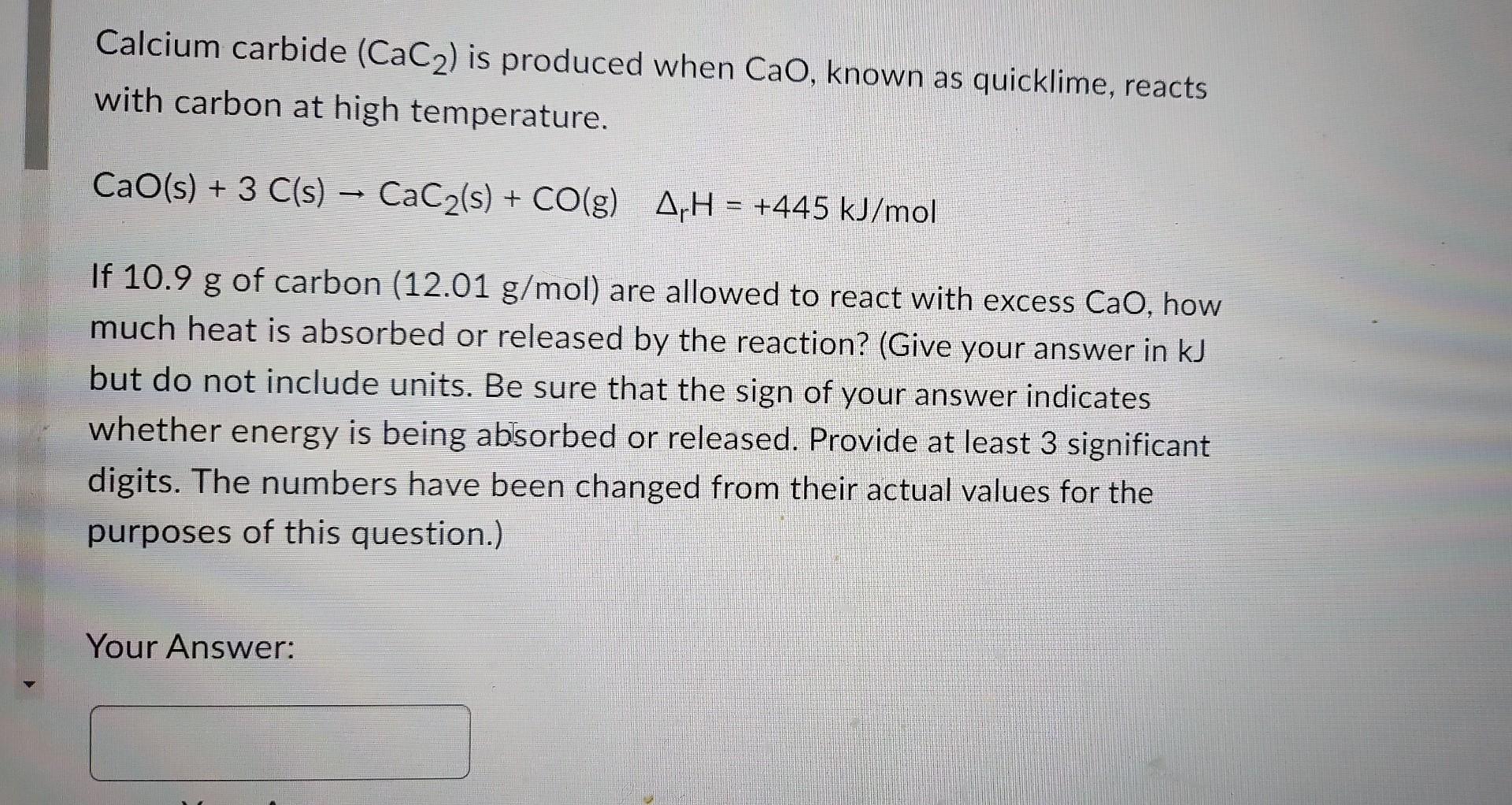
Calcium carbide (CaC2) is produced when CaO, known as quicklime, reacts with carbon at high temperature. CaO(s)+3C(s)CaC2(s)+CO(g)rH=+445kJ/mol If 10.9g of carbon (12.01g/mol) are allowed to react with excess CaO, how much heat is absorbed or released by the reaction? (Give your answer in kJ but do not include units. Be sure that the sign of your answer indicates whether energy is being absorbed or released. Provide at least 3 significant digits. The numbers have been changed from their actual values for the purposes of this question.) Your
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


