Answered step by step
Verified Expert Solution
Question
1 Approved Answer
calculate average rate of reaction for Mg over the whole time. Part 3 (This 'data' iv fil.... We are learning) Mga0)+2HCl(0)MgCl200+H200 a. Calculate the average
calculate average rate of reaction for Mg over the whole time. 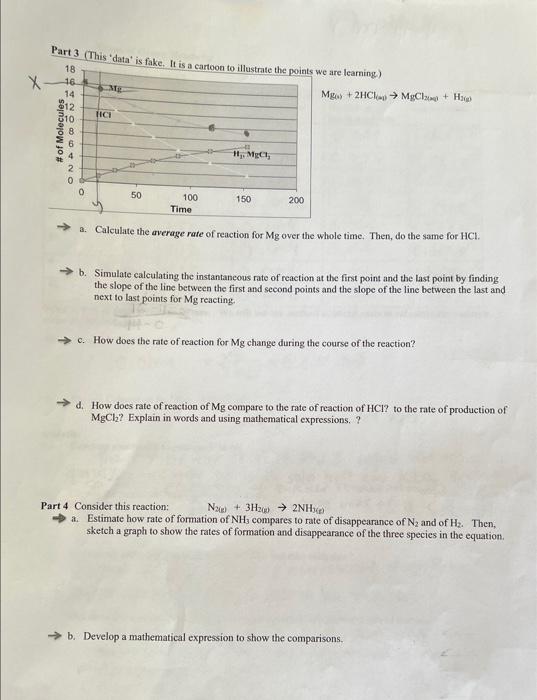
Part 3 (This 'data' iv fil.... We are learning) Mga0)+2HCl(0)MgCl200+H200 a. Calculate the average rate of reaction for Mg over the whole time. Then, do the same for HCl. b. Simulate calculating the instantaneous rate of reaction at the first point and the last point by finding the slope of the line between the first and second points and the slope of the line between the last and next to last points for Mg reacting. c. How does the rate of reaction for Mg change during the course of the reaction? d. How does rate of reaction of Mg compare to the rate of reaction of HCl? to the rate of production of MgCl2 ? Explain in words and using mathematical expressions. ? Part 4 Consider this reaction: N2e+3H2()2NH3(e) a. Estimate how rate of formation of NH3 compares to rate of disappearance of N2 and of H2. Then, sketch a graph to show the rates of formation and disappearance of the three species in the equation. b. Develop a mathematical expression to show the comparisons 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


