Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate delta G^o at 292 K and 318 K and predict the stability of the protein at these temperatures Temp. (K) B Fraction unfolded Fraction
Calculate delta G^o at 292 K and 318 K and predict the stability of the protein at these temperatures 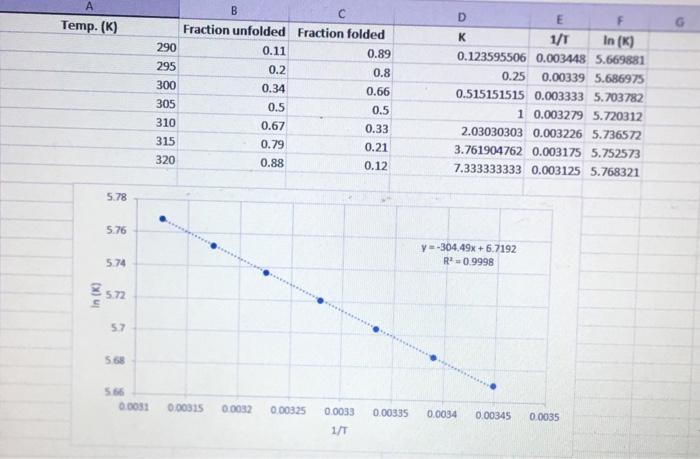
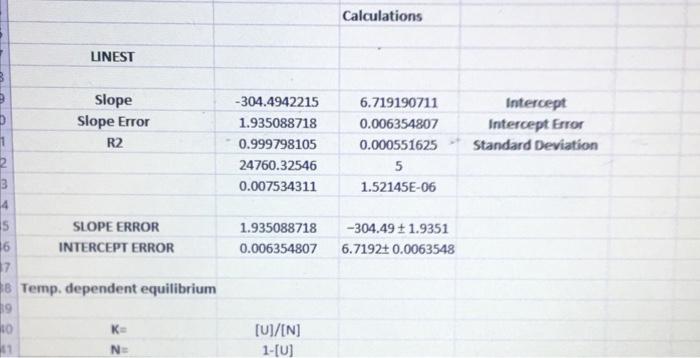
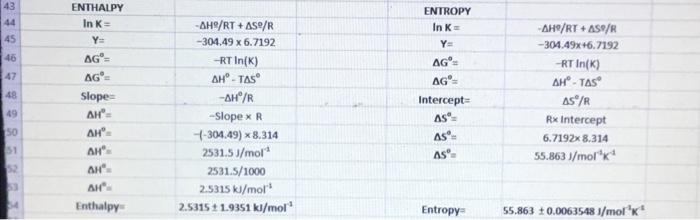
Temp. (K) B Fraction unfolded Fraction folded 290 0.11 0.89 295 0.2 0.8 300 0.34 0.66 305 0.5 0.5 310 0.67 0.33 315 0.79 0.21 320 0.88 0.12 D E K 1/1 In (K) 0.123595506 0.003448 5.669881 0.25 0.00339 5.686975 0.515151515 0.003333 5.703782 1 0.003279 5.720312 2.03030303 0.003226 5.736572 3.761904762 0.003175 5.752573 7.333333333 0.003125 5.768321 5.78 . 5.76 y=-304.49x + 6.7192 R 0.9998 5.74 In (K) 5.72 57 5.68 566 0.0031 0.00315 0.0032 0.00325 0.0033 0.00335 0.0034 0.00345 0.0035 1/T Calculations LINEST Slope Slope Error R2 -304.4942215 1.935088718 0.999798105 24760.32546 0.007534311 6.719190711 0.006354807 0.000551625 5 1.52145E-06 Intercept Intercept Error Standard deviation 1.935088718 0.006354807 -304.49 1.9351 6.7192: 0.0063548 5 SLOPE ERROR 16 INTERCEPT ERROR 17 8 Temp. dependent equilibrium 19 ko K [U]/IN] 1-(0) NE 43 ENTHALPY In K 44 ENTROPY In K= 45 46 47 AGO AG Intercept AS 48 -AH/RT+AS/R -304.49 x 6.7192 -RTIN(K) AH' - Tas -AH/R -Slopex R -304.49) * 8.314 2531.5J/mol 2531.5/1000 2,5315 kJ/mol 2.5315 11.9351 kJ/mol AG AG Slope AH AH' AH AH AH Enthalpy 49 SO 51 -AH/RT + AS/R -304.49x+6.7192 RT In(K AH-TAS AS/R Rx Intercept 6.7192x8.314 55.863 /molk AS as Entropy 55.863 0.00635481/mol K 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


