Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate lattice energy. 2. The lattice energy of an ionic compound is the energy change when one mole of ionic solid is separated into its
Calculate lattice energy.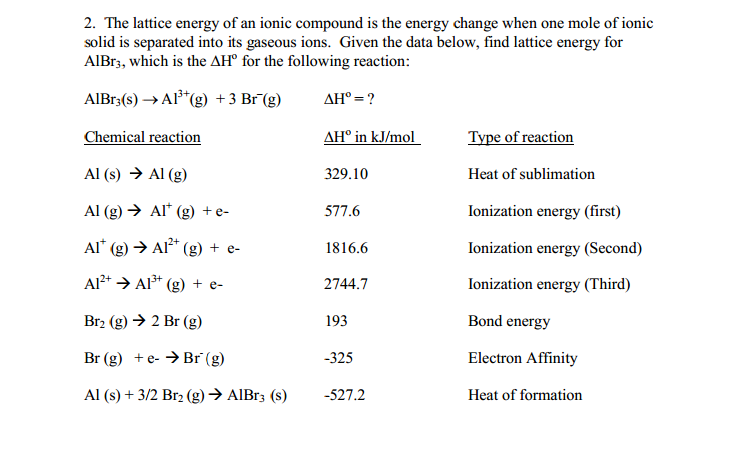
2. The lattice energy of an ionic compound is the energy change when one mole of ionic solid is separated into its gaseous ions. Given the data below, find lattice energy for AlBr3, which is the AH for the following reaction: AlBr3(s) A1+ (g) + 3 Br(g) Chemical reaction Al (s) Al (g) Al (g) Al* (g) + e- Al* (g) Al(g) + e- Al+A1+ (g) + e- Br (g) 2 Br (g) Br (g) + e-Br (g) Al (s) + 3/2 Br (g) AlBr3 (s) AH = ? AH in kJ/mol 329.10 577.6 1816.6 2744.7 193 -325 -527.2 Type of reaction Heat of sublimation Ionization energy (first) Ionization energy (Second) Ionization energy (Third) Bond energy Electron Affinity Heat of formation
Step by Step Solution
★★★★★
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Answer A1 g 3e 3 Br 9 Third ionization enexpy Born Haber ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


