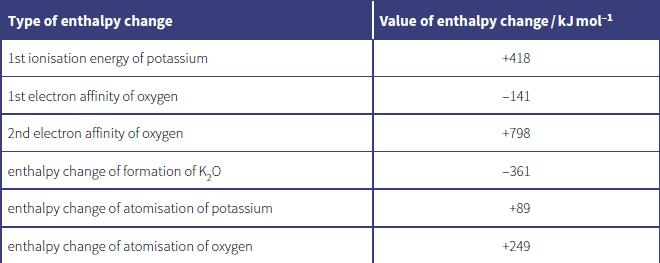
The table shows the enthalpy changes needed to calculate the lattice energy of potassium oxide, K 2
Question:
The table shows the enthalpy changes needed to calculate the lattice energy of potassium oxide, K2O.
a. Copy the incomplete Born–Haber cycle shown below. On the lines A to E of your copy of the Born–Haber cycle, write the correct symbols relating to potassium and oxygen.
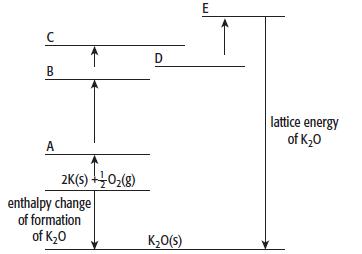
b. Use the data in the table above to calculate the lattice energy of potassium oxide.
c. Describe how, and explain why, the lattice energy of sodium oxide differs from that of potassium sulfide, K2S.
d. Explain why the 2nd electron affinity of oxygen has a positive value.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





