Question
Use the data in the table above to plot In[t-BuCl]; vs time (s). Include labeled axis, a title, and a linear trendline. Upload your plot
Use the data in the table above to plot In[t-BuCl]; vs time (s). Include labeled axis, a title, and a linear trendline. Upload your plot here.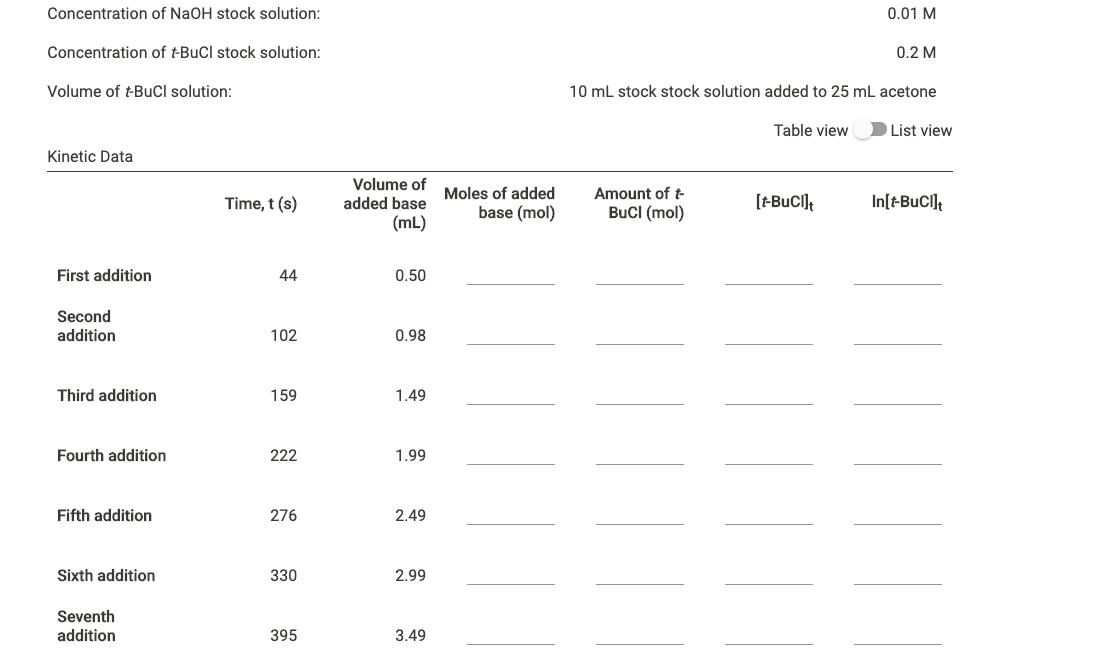
Concentration of NaOH stock solution: 0.01 M Concentration of tBuCl stock solution: 0.2 M Volume of t-BuCl solution: 10 mL stock stock solution added to 25 mL acetone Table view List view Kinetic Data Volume of Moles of added added base Amount of t Time, t (s) base (mol) BuCI (mol) [-Buci] In[-BuCI] (mL) First addition 44 0.50 Second addition 102 0.98 Third addition 159 1.49 Fourth addition 222 1.99 Fifth addition 276 2.49 Sixth addition 330 2.99 Seventh addition 395 3.49
Step by Step Solution
3.37 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Probability And Statistics
Authors: Morris H. DeGroot, Mark J. Schervish
4th Edition
9579701075, 321500466, 978-0176861117, 176861114, 978-0134995472, 978-0321500465
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



