Question
Calculate q, w, AE, and AH, when 255 g of water evaporates at 1.00 atm and 100.0 C. The heat of vaporization of water
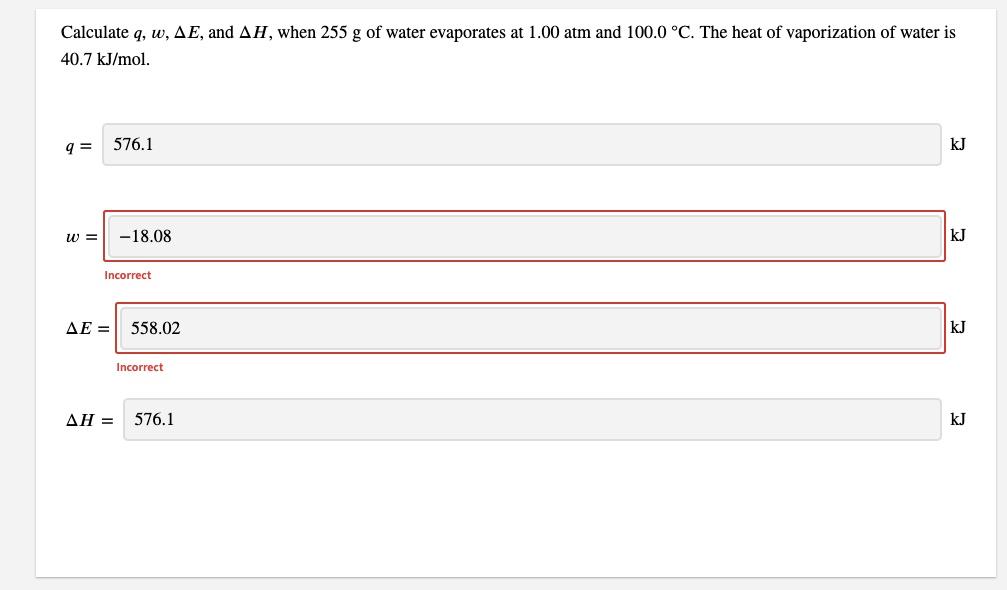
Calculate q, w, AE, and AH, when 255 g of water evaporates at 1.00 atm and 100.0 C. The heat of vaporization of water is 40.7 kJ/mol. 576.1 kJ w = -18.08 kJ Incorrect AE = 558.02 kJ Incorrect AH = 576.1 kJ
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Mass of water 255g No of moles of water weightmolar mass 25518 1416 H...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Mathematical Applications for the Management Life and Social Sciences
Authors: Ronald J. Harshbarger, James J. Reynolds
11th edition
9781337032247, 9781305465183, 1305108043, 1337032247, 1305465180, 978-1305108042
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



