Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the boiling point temperature and vapor phase concentration of the liquid mixture consisting of 0 . 3 i - butane, 0 . 3 2
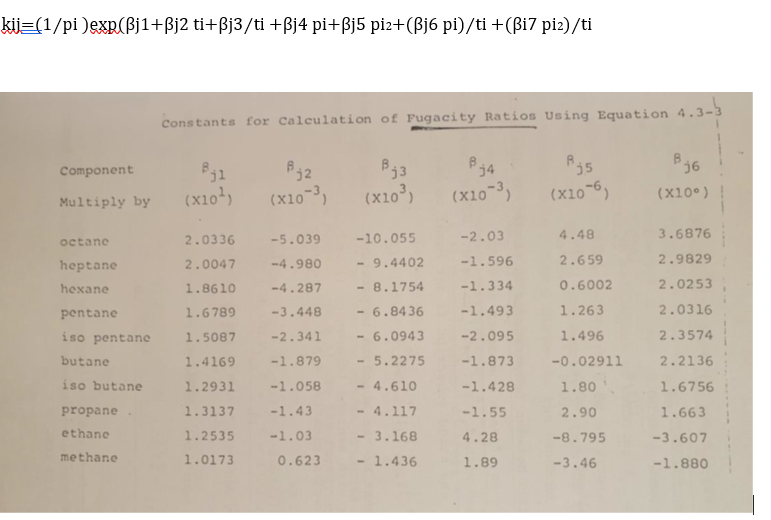
Calculate the boiling point temperature and vapor phase concentration of the liquid mixture consisting of ibutane, nbutane and pentane as mole fractions at atm pressure, manually and using a computer program. Take the first default temperature value as oC Using:
kijpi expbeta jbeta j tibeta jti beta j pibeta j pibeta j piti beta i piti
Constants for Calculation of Fugacity Ratios Using Equation
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


