Question
Calculate the bubble point temperature (TBP, bubble point temp.) at 101.3 kPa of the liquid mixture containing 30% acetone, 30% acetonitrile and 40% nitromethane as
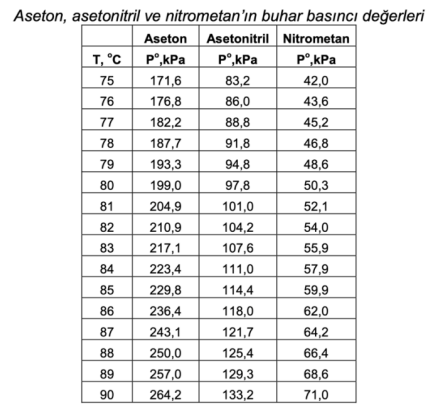
Calculate the bubble point temperature (TBP, bubble point temp.) at 101.3 kPa of the liquid mixture containing 30% acetone, 30% acetonitrile and 40% nitromethane as mole percent and the composition of the vapor phase formed. If the mixture were a vapor mixture containing 30% acetone, 30% acetonitrile and 40% nitromethane in mole percent, what would be the condensation temperature (TDP, dew point temp.) at 101.3 kPa and the composition of the resulting liquid phase?
a) Calculate analytically.
b) Calculate using a computer program.
c) Draw graphs of T versus xacetone, xacetonitrile, xnitromethane, and T versus yacetone, yacetonitrile, nitromethane.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


