Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the compressibility factor of methane propane and ethane at a reduced pressure and temperature of 2,1.6 respectively Use the given fig. to answer Ethane
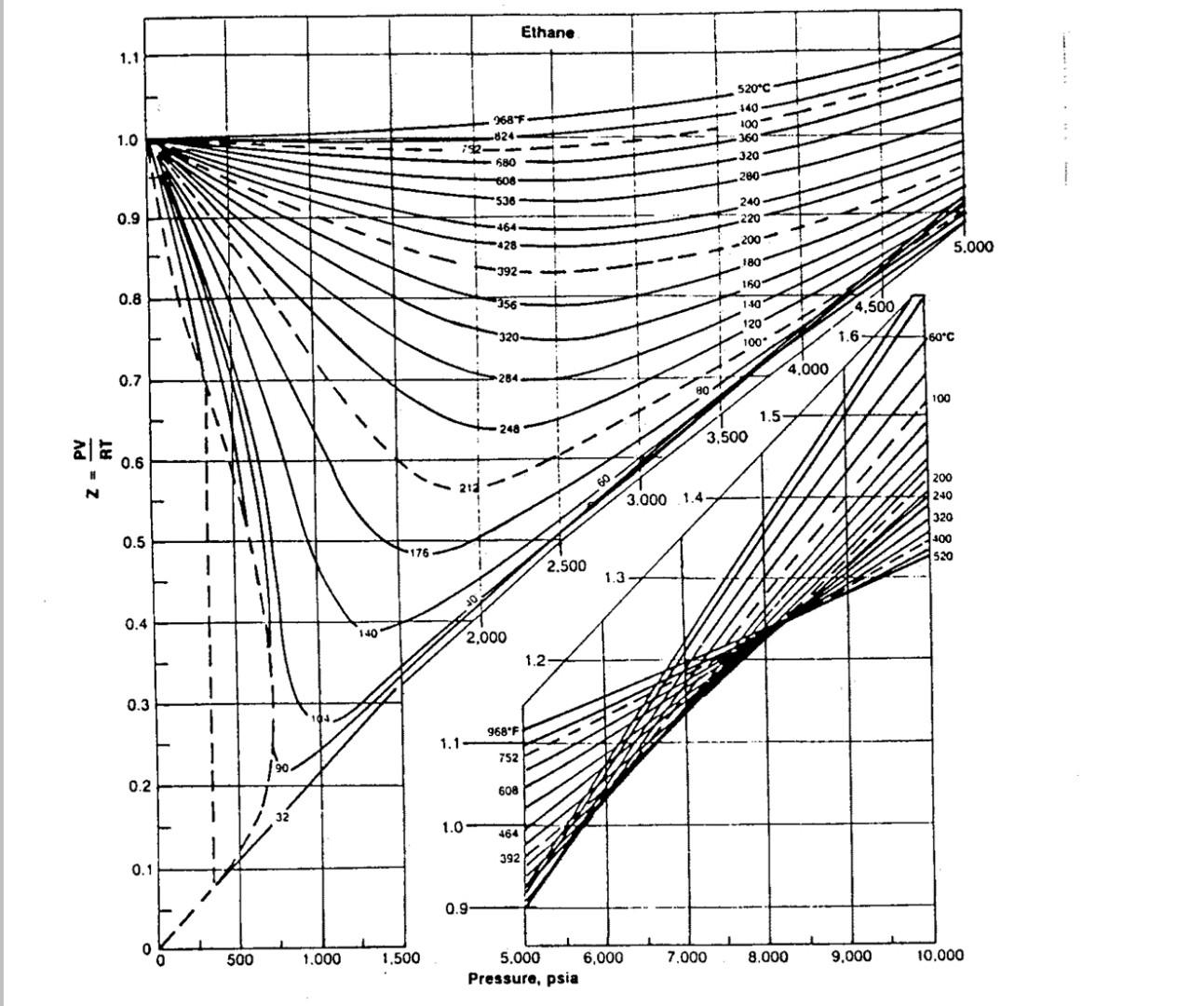
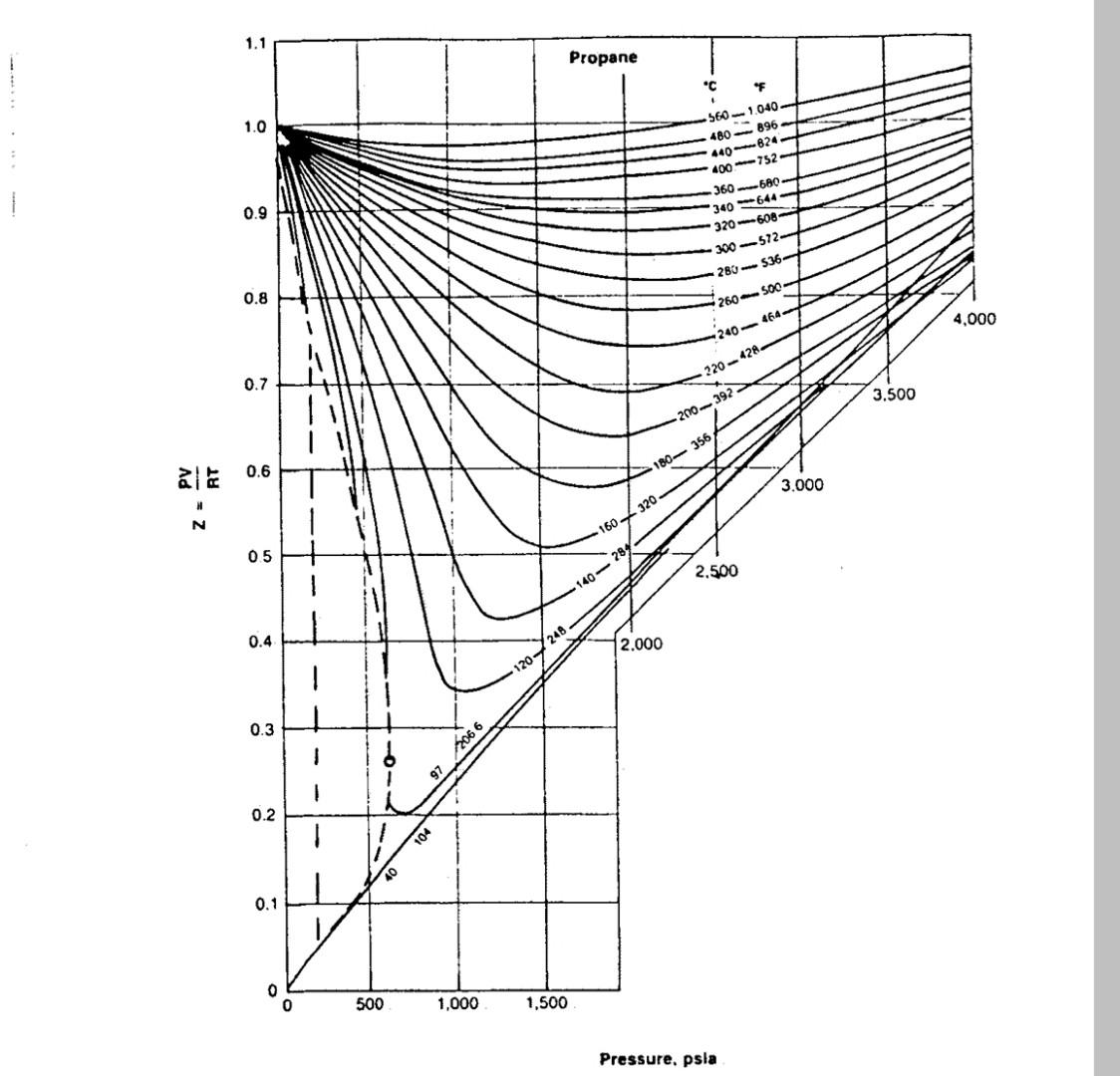
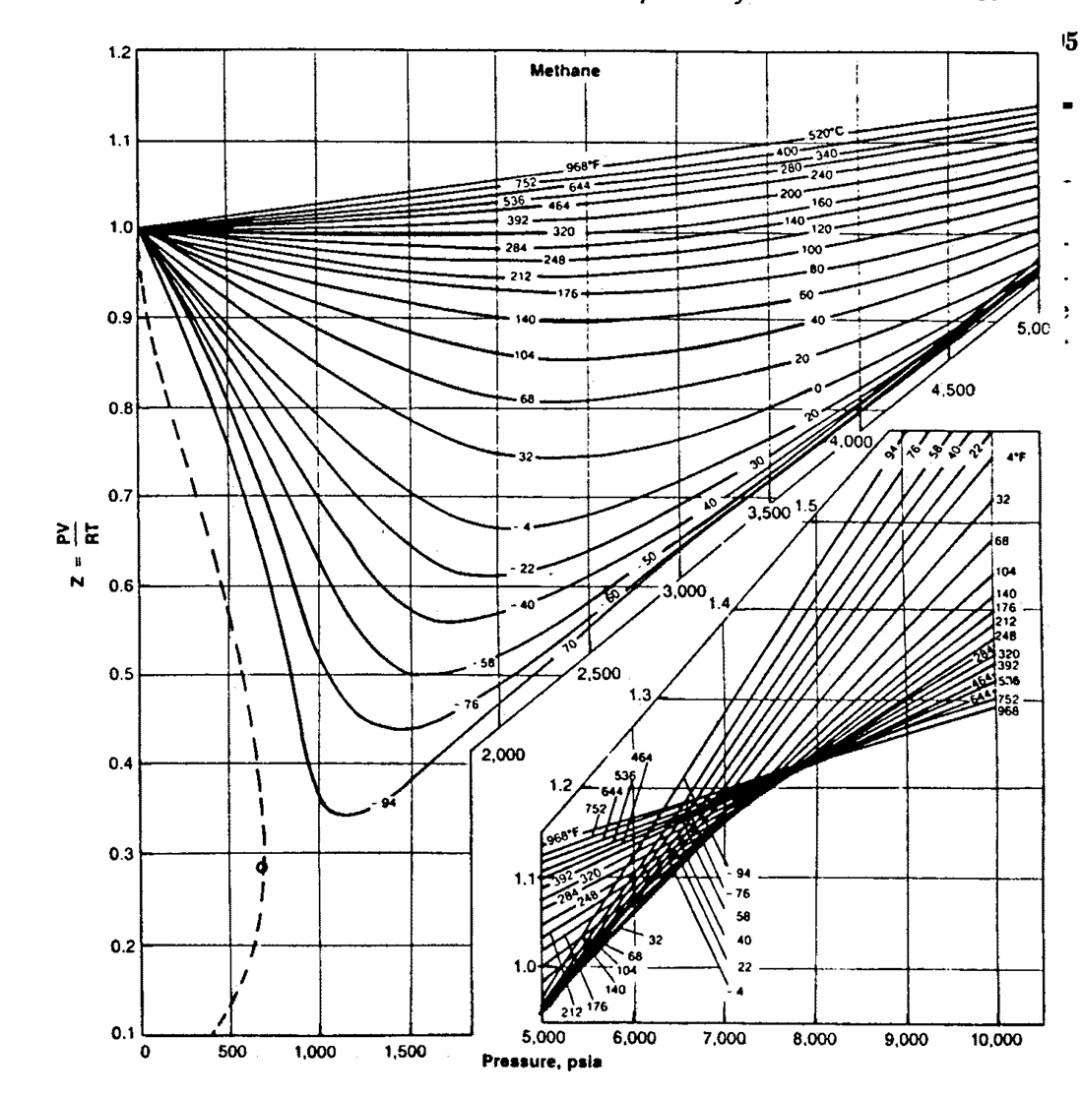
Calculate the compressibility factor of methane propane and ethane at a reduced pressure and temperature of 2,1.6 respectively Use the given fig. to answer
Ethane 1.1 520C 1.0 2685 824 722 580 608 *536 140 100- 360G J20 280 0.9 240 220 464 428 -200 5.000 180 392 150 0.8 156 1-50 4.500) 1.6 120 100* *320 60C 4.000 0.7 284 do 100 1.5 .248 3.500 0.6 N - 3.000 1.47 200 240 320 400 520 0.5 176 2.500 1.3 0.4 140 2.000 1.2 0.3 968 F 1.1 752 90 0.2 608 / 32 1.0 464 392 0.1 0.9 0 0 500 1.000 1,500 7.000 8.000 9,000 10.000 5.000 6,000 Pressure, psia 1.1 Propane "C F 1.0 560 1.040 480896 440624 400-752 360-680 0.9 340 644 * 320-608- 300 - 572 280536 0.8 260 - 500 4,000 240464 220 - 428 0.7 3.500 200-392 180356 ale 0.6 3.000 Z N 160320 05 2.500 140284 0.4 248 2.000 1 0.3 2066 0 97 0.2 TO 0.1 o 500 1,000 1,500 Pressure. psla 15 1.2 Methane - 1.1 520C 400340 9685 752 536 392 644 464 1.0 260 240 200 160 140 120 100 80 320 284 -248 212 -176 60 0.97 140 40 5.00 104 20 4,500 68 0.8 20 4.000 32 0/16 221 4F 30 0.7 32 3.500 1.5 268 104 N 0.6 3.000 1.4 140 176 212 24B 320 392 15.76 752 966 0.5 2.500 1.3 4 464 644 76 0.4 2.000 1.2 464 536 -644 752 968F 0.3 1.1192320 94 76 284 - 24 58 40 32 0.2 68 1.04 22 104 140 212 176 0.1 0 0 6,000 7.000 8.000 9,000 10,000 500 5,000 Pressure, psia 1,000 1,500Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


