Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the concentration of FeSCN2+ for solutions 1-5. Do this using stoichiometry (you can assume the excess SCN1- drives the reaction to completion). Reaction: Fe3+(aq)
Calculate the concentration of FeSCN2+ for solutions 1-5. Do this using stoichiometry (you can assume the excess SCN1- drives the reaction to completion).
Reaction: Fe3+(aq) + SCN-(aq) ⇌ FeSCN2+(aq)
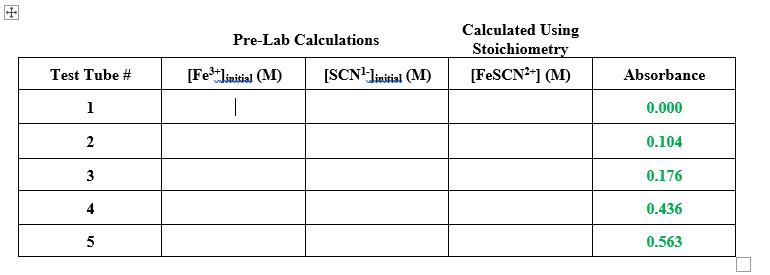
Calculated Using Stoichiometry Pre-Lab Calculations Test Tube # [Fe**linitial (M) [SCN'liaitial (M) [FESCN*"] (M) Absorbance 1 | 0.000 0.104 0.176 4 0.436 0.563 2. 3.
Step by Step Solution
★★★★★
3.47 Rating (170 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635f3cb9db064_229745.pdf
180 KBs PDF File
635f3cb9db064_229745.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


