For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g)
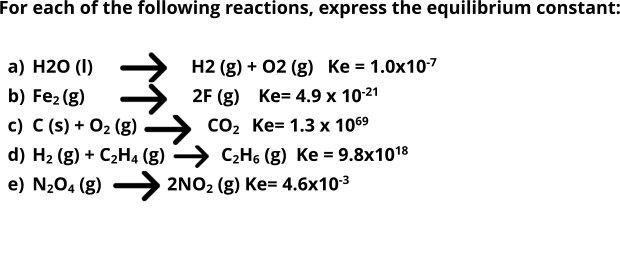
For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g) 2F (g) Ke= 4.9 x 10-21 c) C (s) + O2 (g) d) H2 (g) + C2H4 (g) C2H6 (g) Ke = 9.8x1018 e) N204 (g) - 2NO2 (g) Ke= 4.6x103 CO2 Ke= 1.3 x 1069
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Equi librium Cosfart is erpressed as the eqilibriun Con... View full answer

Get step-by-step solutions from verified subject matter experts
100% Satisfaction Guaranteed-or Get a Refund!
Step: 2Unlock detailed examples and clear explanations to master concepts

Step: 3Unlock to practice, ask and learn with real-world examples

See step-by-step solutions with expert insights and AI powered tools for academic success
-
 Access 30 Million+ textbook solutions.
Access 30 Million+ textbook solutions.
-
 Ask unlimited questions from AI Tutors.
Ask unlimited questions from AI Tutors.
-
 Order free textbooks.
Order free textbooks.
-
 100% Satisfaction Guaranteed-or Get a Refund!
100% Satisfaction Guaranteed-or Get a Refund!
Claim Your Hoodie Now!

Study Smart with AI Flashcards
Access a vast library of flashcards, create your own, and experience a game-changing transformation in how you learn and retain knowledge
Explore Flashcards





