Explain why one of these carbocations is much more stable than the other: + CH-CH3 CH CH
Question:
Explain why one of these carbocations is much more stable than the other:
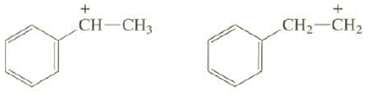
Transcribed Image Text:
+ CH-CH3 CH₂ CH₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
The p orbital of the positively charged carbon of the carbocation on the left is con...View the full answer

Answered By

Cristine kanyaa
I possess exceptional research and essay writing skills. I have successfully completed over 5000 projects and the responses are positively overwhelming . I have experience in handling Coursework, Session Long Papers, Manuscripts, Term papers, & Presentations among others. I have access to both physical and online library. this makes me a suitable candidate to tutor clients as I have adequate materials to carry out intensive research.
4.90+
1538+ Reviews
3254+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain why one of these anions is much more stable than the other: : a) CH-C-CH-CH b) CH CH3 : CH-C-CH-CH CH-C=N:
-
One of the following compounds is much more stable than the other two. Classify each as aromatic, antiaromatic, or nonaromatic. heptalene azulene pentalene
-
Explain why one of these compounds reacts readily by an E2 mechanism when treated with sodium ethoxide in ethanol but the other doesnot: OTs OTS . H, .H, CH; CH3
-
Amrito Corporation is under financial distress and raises debt because it has several projects that are expected to generate profit in the future. When calculating its weighted average cost of...
-
Define the fixed overhead budget variance, and explain how it may assist manager control costs?
-
Radford Corporation's charter authorized 1 million shares of $11 par value common shares, and 300,000 shares of 6% cumulative and non-participating preferred shares, with a par value of $100 per...
-
8. Under what circumstances would the direction of intercompany inventory transactions not affect the allocation of unrealized profit?
-
The JM Partnership was formed to acquire land and subdivide it as residential housing lots. On March 1, 2014, Jessica contributed land valued at $600,000 to the partnership in exchange for a 50%...
-
In the cost equation TC = F + VX, "V" is best described as the: Multiple Choice total costs that do not vary with changes in the activity level. intercept of the cost equation. variable cost per...
-
A wind turbine with two or four hollow hemispherical cups connected to a pivot is commonly used to measure wind speed. Consider a wind turbine with four 8-cm-diameter cups with a center-to-center...
-
Explain why this carbocation is considerably more stable than this structure would suggest: H +C-0-CH, H
-
Show energy level diagrams for the MOS of these compounds: a) H-C=C-CH3 b) CH0H c) CH3-C-H
-
Methanol (CH3OH) has also been proposed as an alter-native fuel. Calculate the standard enthalpy of combustion per gram of liquid methanol, and compare this answer to that for ethanol in Exercise 80.
-
15.5 please help will give like if answers r correct Exercise 15-8 (Static) Sales-type lease with selling profit; lessor; calculate lease payments [LO15-3] Manufacturers Southern leased high-tech...
-
When my son was young, he had 8 different plastic dinosaurs to arrange. How many ways could he arrange his 8 dinos? He had favorite dinos, so placing them in proper order was very important. How many...
-
Process P1 init (mutEx); num = 0; loop1 = 0; while (loop1 < 3) wait (mutEx); num num + 1; signal (mutEX); loop1 loop1 + 1; Process P2 loop2 = 0; while (loop2 < 2) wait (mutEx); num num + 10;...
-
PROBLEM 3-5B Following is the chart of accounts of Smith Financial Services: Assets 111 Cash 113 Accounts Receivable 115 Supplies 117 Prepaid Insurance 124 Office Furniture Liabilities 221 Accounts...
-
4. Identify a service you could refer Casey to and write a referral for her (up to 300 words).
-
Describe a situation where you were very aware of the type of need that was motivating your behavior
-
Using Gauss-Jordan elimination, invert this matrix ONLY 0 0 0 0 1
-
Find the sum of the geometric series 5 10/3 + 20/9 40/27 + .....
-
Name the following substances, including the cis- or trans-prefix: CH2CH3 (b) , (a) H. - - CI
-
Draw the structures of the following molecules: (a) Trans-1-Bromo-3-methylcyclohexane (b) Cis-1, 2-Dirnethylcyclobutane (c) Trails-1-tert-Butyl-2-ethylcyclohexane
-
Prostaglandin F2?, a hormone that causes uterine contraction during childbirth, has the following structure. Are the two hydroxyl groups (?OH) on the cyclopentane ring cis or trans to each other?...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not
-
Explain: An office building is renting for $10/sf, with 50,000 total leasable square feet. Office buildings in the area are selling for cap rates of 5.5%. What information do you have and what are...

Study smarter with the SolutionInn App


